MRI Gadolinium Toxicity - Untold Danger
to Patients Worldwide
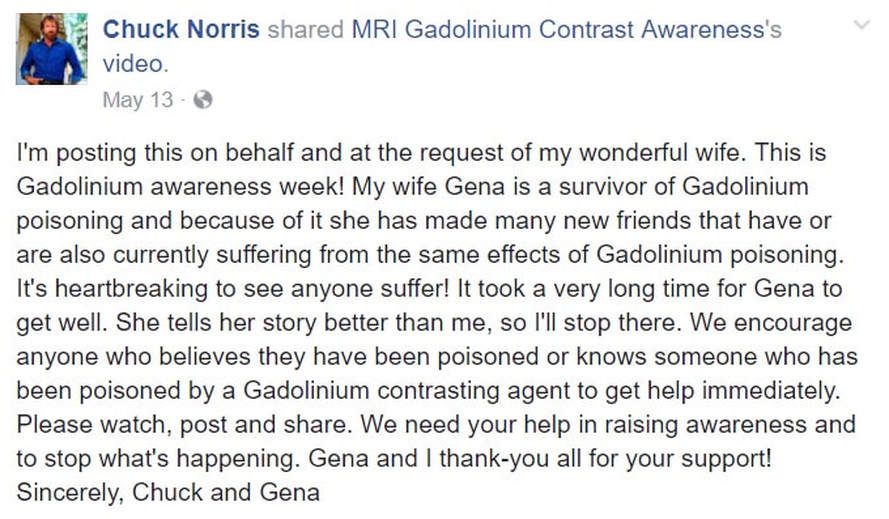
MRIs are a powerful tool to diagnose and track disease but there's concern that the contrast dyes sometimes used may be dangerous for some. Chuck Norris and his wife discuss what happened after Gena Norris had three MRIs in one week.
I have had conversations with my Neurologist and my new Natural Health 'Aware' PCP. We couldn't figure out why I was regressing in certain areas of my health. I was using Oral Chelation Therapy....my idea. My doctor knows and he ordered the required labs to monitor my progress. Plus, I take the necessary health supplements to keep my mineral lab values within normal ranges. Nope, my doctors didn't have a clue. They couldn't tell me what was causing the regression, but I felt like I had Metal Toxicity...Yes, I'm always researching and that fits my symptoms. I tell you, doctors don't know everything. You have to be your own health advocate.
I was feeling crappy and took a day off. In the afternoon I decided to do some more research. Look at what I found...Holy Cow...
MRAs...MRI done with the Gadolinium IV contrast, is causing Gadolinium Toxicity.
The FDA knows about it, other countries have discontinued using Gadolinium in humans, but the good old USA refuses to ban the use of this toxic substance in humans. So, whether you have healthy kidney function or not, you will be subjected to this poison, if you do not advocate for yourself and refuse it...next time you must have an MRI imaging exam ordered to be done with contrast.
I remember the last MRI with contrast I had. I was an inpatient for a terrible bout of Vertigo, and I told my nurse that something was wrong with the contrast fluid because it made me feel weird. He thought I was making a joke, so I just left it at that. I was imagining all kind of things and was even wondering if I got a bad batch of the contrast.
Today, after seeing this research, I called the hospitals to check the name of the contrast that they used during my MRI imaging procedures...Yup, they both used Gadolinium. I know I was right. I had a reaction, because I was already suffering from Gadolinium toxicity, and getting another dose of that poison, made me feel the effects immediately. Gadolinium is medication. I had an adverse reaction to that medicine but no one noticed or cared because it is considered 'safe.'
European Medicines Agency takes action on Linear Gadolinium-based Contrast Agents
How many MRA's have I had since 2004, the year I had the TBI? Plenty...The last one was less than a year ago. I have an order for one due before October 5. You know that is not going to happen now that I found this important research. How many MRAs have you had? Do you see any of your nagging chronic symptoms on the Symptoms list? Check out the list below. You may find the reason for you illness that your doctor may have told you, is all in your head. Well, now I know I was right. It was toxicity. The Oral Chelation did make me feel better. Now I will have a different kind of conversation with my doctors.
I have been doing Oral Chelation thinking my perceived metal toxicity was caused by vaccines...Now I know it may have been a combination of both vaccines and Gadolinium Toxicity. There is a toll free number to report suspected Gadolinium Toxicity to the FDA. Imagine that. Why all the secrecy? Here's the info to report to the FDA ...
Have you been affected by retained Gadolinium from a contrast MRI?
Anyone who has unexplained symptoms (See symptoms below) that you believe were caused by retained Gadolinium from a contrast MRI or MRA should report it to the FDA by filing a MedWatch Adverse Event Report. Call 1-800-FDA-1088 or report via the FDA website at http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm
When filing a report online, remember that Gadolinium-Based Contrast Agents are considered prescription medications or drugs.
It is important that Adverse Event Reports related to Gadolinium-Based Contrast Agents are filed with the FDA or the full scope of Gadolinium-related health problems may never be brought to light.
Patients outside the U.S. report to their country’s equivalent governing agency.
I guess when they have a big enough data base with individuals suffering from Gadolinium Toxicity, they may do something...like have official tests to diagnose it in a reasonable period of time?
I had also bought Liquid Zeolite from this company for my daughter to use for metal toxicity, during her cancer treatment.
Recent Viewpoints
I was feeling crappy and took a day off. In the afternoon I decided to do some more research. Look at what I found...Holy Cow...
MRAs...MRI done with the Gadolinium IV contrast, is causing Gadolinium Toxicity.
The FDA knows about it, other countries have discontinued using Gadolinium in humans, but the good old USA refuses to ban the use of this toxic substance in humans. So, whether you have healthy kidney function or not, you will be subjected to this poison, if you do not advocate for yourself and refuse it...next time you must have an MRI imaging exam ordered to be done with contrast.
I remember the last MRI with contrast I had. I was an inpatient for a terrible bout of Vertigo, and I told my nurse that something was wrong with the contrast fluid because it made me feel weird. He thought I was making a joke, so I just left it at that. I was imagining all kind of things and was even wondering if I got a bad batch of the contrast.
Today, after seeing this research, I called the hospitals to check the name of the contrast that they used during my MRI imaging procedures...Yup, they both used Gadolinium. I know I was right. I had a reaction, because I was already suffering from Gadolinium toxicity, and getting another dose of that poison, made me feel the effects immediately. Gadolinium is medication. I had an adverse reaction to that medicine but no one noticed or cared because it is considered 'safe.'
European Medicines Agency takes action on Linear Gadolinium-based Contrast Agents
How many MRA's have I had since 2004, the year I had the TBI? Plenty...The last one was less than a year ago. I have an order for one due before October 5. You know that is not going to happen now that I found this important research. How many MRAs have you had? Do you see any of your nagging chronic symptoms on the Symptoms list? Check out the list below. You may find the reason for you illness that your doctor may have told you, is all in your head. Well, now I know I was right. It was toxicity. The Oral Chelation did make me feel better. Now I will have a different kind of conversation with my doctors.
I have been doing Oral Chelation thinking my perceived metal toxicity was caused by vaccines...Now I know it may have been a combination of both vaccines and Gadolinium Toxicity. There is a toll free number to report suspected Gadolinium Toxicity to the FDA. Imagine that. Why all the secrecy? Here's the info to report to the FDA ...
Have you been affected by retained Gadolinium from a contrast MRI?
Anyone who has unexplained symptoms (See symptoms below) that you believe were caused by retained Gadolinium from a contrast MRI or MRA should report it to the FDA by filing a MedWatch Adverse Event Report. Call 1-800-FDA-1088 or report via the FDA website at http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm
When filing a report online, remember that Gadolinium-Based Contrast Agents are considered prescription medications or drugs.
It is important that Adverse Event Reports related to Gadolinium-Based Contrast Agents are filed with the FDA or the full scope of Gadolinium-related health problems may never be brought to light.
Patients outside the U.S. report to their country’s equivalent governing agency.
I guess when they have a big enough data base with individuals suffering from Gadolinium Toxicity, they may do something...like have official tests to diagnose it in a reasonable period of time?
I had also bought Liquid Zeolite from this company for my daughter to use for metal toxicity, during her cancer treatment.
Recent Viewpoints
Safe Chelation for Gadolinium & Companion Heavy Metals
Background on Gadolinium
What is Gadolinium?
Gadolinium is a Rare Earth Element (REE) with paramagnetic properties. On the Periodic Table its symbol is Gd and its atomic number is 64.[1],[2] Gadolinium is one of 15 metallic chemical elements known as the Lanthanide Series. Lanthanides have been referred to as “bone seekers” because they tend to deposit in bone.[3], [4] Tests have confirmed that Gadolinium deposits in bone where it can remain for many years.[5],[6],[7],[8]
Gadolinium (Gd3+) has an ionic radius very similar to that of Calcium (Ca2+) which is why Gd3+ is so toxic in biological systems – Gd3+ can compete with Ca2+ in all biological systems that require Ca2+ for proper function and, in doing so, the trivalent Gd3+ ion binds with much higher affinity.[1]
Gadolinium is toxic to mammals and it has no known biological use in the human body. [9],[10],[11],[12],[13],[14],[15]
Gadolinium-Based Contrast Agents are used for MRI and MRA.Gadolinium has several specialized uses, but we will focus on its paramagnetic properties. In the early 1980s contrast agent developers determined that Gadolinium’s paramagnetic properties could be used to enhance images of abnormal tissue on Magnetic Resonance Imaging (MRI) scans.[16],[17]
Since free Gadolinium is toxic, the Gadolinium ion must be chelated or bound to a ligand (molecule) before it can be used as a contrast agent. The resulting complex is a Gadolinium-Based Contrast Agent or GBCA which is injected intravenously during a contrast-enhanced MRI.[18] GBCAs are also used for contrast-enhanced Magnetic Resonance Angiography (MRA). (Note that MRI and MRA are also performed without contrast.)
The intravenously administered GBCA should move rapidly through the body and then be excreted primarily via the kidneys before the toxic
Gadolinium ion and ligand can separate.[19] With normally functioning kidneys, most of the GBCA should be excreted in less than two hours after injection with the remainder being excreted over the next few days. However, impaired kidney function causes the GBCA to remain in the body for much longer periods of time which can result in the separation of the contrast agent and retention of the toxic Gadolinium ion.
In their 1984 report “Gadolinium-DTPA as a Contrast Agent in MRI: Initial Clinical Experience in 20 Patients”, Carr et al warned that “care should obviously be taken in patients with impaired renal and/or hepatic function where high in vivo concentrations of Gd-DTPA may occur for prolonged periods”.[20] Gd-DTPA, also known as Magnevist, was the first Gadolinium-Based Contrast Agent and it was approved by the FDA in 1988. (See Background on GBCAs for details.)
When severe skin problems first appeared in 1997 in a group of dialysis patients,[21] the connection was not made to that initial warning about the use of Gadolinium-Based Contrast Agents in renally-impaired patients. It wasn’t until 2006 that retained Gadolinium from GBCAs was confirmed as the probable cause.[22],[23]
When Gadolinium is retained in the body, it can have serious consequences – the most serious being an incurable and potentially life-threatening disease known as Nephrogenic Systemic Fibrosis or NSF which is thought to primarily affect patients with severely impaired kidney (renal) function. It is widely recognized by the medical community and government agencies that the toxicity of retained Gadolinium is the primary contributor to the development of NSF.
NSF was first thought to be a skin disease, but it was later learned that retained Gadolinium adversely affects all body systems by causing extensive fibrosis and calcification of connective tissue and internal organs. (See Background on NSF for details.)
Patients exposed to GBCAs are at risk of retaining Gadolinium.
The risks associated with the administration of Gadolinium-Based Contrast Agents to patients with severely impaired kidney function (eGFR <30) are well-documented and recognized by the medical community and FDA. Patients with normal kidney function are not thought to be at risk of retaining Gadolinium from GBCAs; however, published literature indicates that some Gadolinium from each dose of contrast may remain in the body of all patients exposed to GBCAs.[8], [24]
A 1991 article by Rocklage et al titled “Metal Ion Release from Paramagnetic Chelates: What is Tolerable?” reported that “minute amounts of chelated or unchelated metals are likely to remain in the body for an extended period and could possibly result in a toxic effect.” They also said it was “unlikely that MRI contrast agents would be administered repeatedly in patients”.[25] Unfortunately, many patients do have multiple MRI scans with contrast which can result in more Gadolinium remaining in the patient’s body.
Researchers have estimated that approximately 1% (15 mg) of the 1.5 grams of injected Gadolinium from each dose of contrast (0.1 mmol/kg body weight) may be released from the contrast agent and deposited in the bones of GBCA exposed patients including those with normal kidney function.[26]
The long-term and cumulative effects of retained Gadolinium are unknown as are the additive effects of the double or triple doses often used for contrast MRAs.
Besides severely impaired kidney function, there are other factors that can increase the risk of retaining Gadolinium. Those risk factors include acidosis, transient acute kidney injury (AKI), recent surgery, inflammatory events, abnormal vascularity, compromised blood-brain barrier, cumulative dosage, and transmetallation.[27],[28],[29],[30] Other than kidney impairment, researchers have found that transmetallation presents the greatest potential risk for the release of the toxic metal ion from the chelate.[25], [31] Transmetallation is a potential risk for every GBCA exposed patient. (See Risk Factors for more details.)
Gadolinium can deposit in brain tissue.
MRIs with contrast are frequently performed when the brain is being imaged. The brain is protected from potentially harmful compounds in the blood by the semi-permeable Blood-Brain Barrier (BBB). The intact BBB protects the brain from damage, whereas the dysfunctioning BBB allows influx of normally excluded hydrophilic molecules into the brain tissue.[32]
A brain tumor or lesion causes a disruption or break in the BBB. That “break” gives the Gadolinium-Based Contrast Agent access to the brain – regardless of the patient’s level of renal function at the time of his or her contrast procedure. So...TBI survivors and Stroke survivors who undergo multiple MRI's with contrast are at risk of Gadolinium Toxicity...Yep. I'm in that group...TBI Survivor.
GBCA product labeling indicates that Gadolinium-Based Contrast Agents “do not cross an intact blood-brain barrier”; however, “disruption of the blood-brain barrier” or “abnormal vascularity” allows accumulation in lesions such as neoplasms (tumors), abscesses, and subacute infarcts.[33],[34],[35],[36],[37],[38],[39],[40] That is why Gadolinium is deposited in brain tumors and brain lesions such as those seen in Multiple Sclerosis. The abnormal brain tissue then becomes more clearly defined or enhanced on magnetic resonance (MR) images.[41] Gadolinium should not remain in the body; however, the literature indicates that Gadolinium retention has occurred.
A 1995 case report by Martinez noted persistent Gadolinium retention in an extra-axial brain stem lesion of a patient with Erdheim-Chester Disease. Gadolinium retention was confirmed by comparing precontrast and postcontrast images from two MRIs performed 23 days apart.[42] A 1989 case report of Erdheim-Chester disease reported persistent enhancement of intracerebral lesions 8 days after injection with Gd-DTPA. Chemical analysis of the biopsy specimen revealed a high concentration of Gadolinium.[43]
A 2009 study by Fulciniti et al found that a Gadolinium-containing contrast agent promoted Multiple Myeloma (MM) cell growth. Autopsies of 8 MM patients with repeated exposure to the agent found “massive amounts of Gadolinium accumulation in tissues regardless of their renal function”.[44]
A 2010 study by Xia et al found insoluble deposits containing Gadolinium in 7 brain tumor biopsies from 5 patients whose eGFRs were above 53 ml/min, confirming that brain tumors alter the Blood-Brain Barrier which allows the injected GBCA to deposit in brain tissue, even in patients without severe kidney problems.[45]
A 2013 study by Kanda et al found that abnormally high signal intensity detected in two regions of the brain on unenhanced T1-weighted images was related to the number of previous Gadolinium-based contrast enhanced MRIs that had been undergone by the population of patients with normal renal function.[46]
Patients with Multiple Sclerosis (MS) routinely have MRIs with contrast to monitor disease activity and response to treatment.
Gadolinium will enhance “active” MS lesions due to a breakdown in the Blood-Brain Barrier.[47] Serial contrast MRIs have shown that active lesions generally enhance for a period of 1 to 2 months on average.[48],[49]The enhancing lesion evolves to a chronic T2 hyperintense lesion, which is the non-specific ‘footprint’ of the prior inflammatory event.[50] It has been speculated “that the initial enhancing-inflammatory lesion events in the brain, place into motion, at an early stage, the processes that ultimately lead to cerebral atrophy, and these processes may include early axonal injury”.[50]
While the role of enhancing-inflammatory lesions in the development of cerebral atrophy in MS is unclear, studies have demonstrated that cerebral atrophy paralleled that of contrast enhancing lesion accumulation.[51],[52] As noted by Simon, atrophy, particularly that resulting from volume loss around the third ventricle, appears to be predicted by the presence of enhancing lesions at baseline.[50]
Patients without enhancing lesions at baseline showed no increment (increase) in third ventricle width, while the enhancing group showed significant increments in atrophy.[50] The enhancement is the result of deposited Gadolinium; however, the literature does not indicate whether or not Gadolinium deposition or retention leads to brain atrophy.
Retention of Gadolinium from contrast MRIs has been confirmed in a patient with MS. Results of provoked urine testing for toxic metals after administration of the chelating agent EDTA showed high levels of Gadolinium.[53] The chelating agent can extract elements from tissue in much higher quantity than would normally be excreted in urine. Removal of Gadolinium and other metals by chelation therapy was reported to definitively improve the patient’s MS symptoms.
While the presence of a brain tumor or lesion alters the Blood-Brain Barrier, there are other ways that the BBB might be crossed or temporarily altered, such as the following:
Note that findings of delayed and hyperintense enhancement of Cerebrospinal Fluid (CSF) have been reported in patients with normal renal function.[62],[63],[72],[73],[74]
Here are other important facts about Gadolinium and GBCAs.
The FDA and medical industry directly involved with NSF and GBCAs are aware of these facts. However, clinicians have been told that patients with normal kidney function do not retain Gadolinium and are therefore not at risk from a contrast-enhanced MRI or MRA. Because of that, Gadolinium-related health problems may be greatly under reported.
Gadolinium and MRIs: A Common, Risky Connection
When patients get MRIs, they most commonly hear that they will be injected with a “dye” that will help the hospital staff read the results. What they are not told is that in about 30% of cases, this “dye” is actually gadolinium, an extremely poisonous metal.
The medical community thinks that it is pretty harmless, and that it leaves the body right after an MRI is done. As we see from Gena’s horrible experience, that is not always the case.
The internal FDA documents show that many scientists have known for over a decade that this issue exists, yet nothing has been done to protect millions of people who get MRIs every year.
(About 30 million Americans get an MRI every year; that’s one in every 10 people. Three countries that administer even more MRIs than the U.S. are Japan, Germany and Turkey)
In 2006, the FDA recognized that there is a strong link between gadolinium and nephrogenic systemic fibrosis (NSF), a deadly condition that leads to thickening of organs and skin.
In 2007, they had to add a warning to gadolinium stating that it can put patients who have weak kidneys at risk as their bodies might not be able to eliminate this toxin from their bodies.
The first problem is that many people and their doctors have no idea that they have weak kidneys.
The second problem is this is not the case with every patient.
Sharon Williams’s health changed for the worst after her 5th MRI. (founder of www.GadoliniumToxicity.com)
After Gena, Full Measure host interviewed Sharon Williams, a patient advocate for gadolinium poisoning and one of the two founders of GadoliniumToxicity.com
Sharon had five MRIs in her life, and after each one her health deteriorated more and more. However, she has zero kidneys problems, which shows that gadolinium can be toxic to people with functioning kidneys as well.
A test has revealed that she had this heavy metal stored in her thyroid gland and brain. After MRIs she developed a rash and other skin problems, which made her lose hair on her legs. She had symptoms in her abdomen, her ribs, and her head. She was in pain. She had muscle spasms, high blood pressure, and poor cognitive function.
After realizing the connection between her health struggles and gadolinium, Sharon created GadoliniumToxicity.com together with Hubbs Grimm to educate patients. She has collected 500 research articles about gadolinium and its toxicity, and now fights for appropriate treatment for all who are affected by this heavy metal.
Modern medical treatment for gadolinium poisoning is highly expensive and is difficult to find. The Norris family spent over $2 million on Gena’s treatment. This is not something many families can afford.
“We’ve been blessed enough to be able to afford the alternative or the integrative treatments and modalities and the medicines that are out there. There are millions of people that, that don’t have that, okay, and because insurance doesn’t pay,” Gena said.
Pharmaceutical Companies’ Grip on Gadolinium
The FDA has so far done nothing about the gadolinium issue. But the information is slowly penetrating the public and affecting government policy. Last year in Europe, a government health committee made a recommendation that gadolinium should be suspended from use, even as it is still routinely administered to patients here in the U.S.
One of the major companies that makes it, General Electric’s (GE) health care division, asked officials to reconsider the ban. General Electric, pharmaceutical company Bayer, and the FDA all claim that “the risk benefit profile [for gadolinium products] remains favorable.”
Instead of banning this chemical, the FDA approved it for use for infants and babies under two years old. It plans to hold a public meeting to discuss the gadolinium issue in the future.
By means of a symptom survey of 17 people with high urine levels of Gadolinium, we have provided a comprehensive review of this topic in Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs, which we encourage you to read. On this page, we will provide some high-level information from that paper as well as other observations we have gathered from MRI Gadolinium Support Group.
Getting Gadolinium Toxicity from Contrast MRIs
When undergoing an MRI with contrast, a Gadolinium-Based Contrast Agent (GBCA) is injected into your vein. All of the people who order and administer the GBCA believe that it is totally safe for people with normally functioning kidneys and that it will be gone from your body within a few days via your urinary tract.
Many, many people have received contrast MRIs with no adverse effects that they have identified as related to or caused by the contrast agent. But we, ourselves, and others active in the Support Group and participants in the original research that we conducted, feel strongly that we have been adversely affected by retained Gadolinium from the contrast administration.
Although there has been no published direct measurement of retained Gadolinium, it has been estimated that 1% of the injected Gadolinium from each dose of contrast is retained in the body. Our research supports the idea that Gadolinium is retained as evidenced by urine testing for Gadolinium.
In our Study of Retained Gadolinium from Contrast MRIs report, we document urinary Gadolinium levels in 24-hour collections that show Gadolinium retention well beyond three days.
Based on much of the published medical research on GBCAs and NSF, the basic problem appears to be that the toxic Gadolinium ion separates from the chelate (ligand) to which it was bound in the manufacturing process in order to reduce the toxicity of the metal ion; it is postulated that the longer the GBCA remains in the body, the greater the likelihood of this separation. This is supported by the predominance of NSF patients having impaired renal function, which caused the GBCA to remain in the body longer.
Other than renal insufficiency, no one knows all the co-factors that may cause one person to retain or react to Gadolinium while another person does not. However, the following may increase the risk of Gadolinium Toxicity regardless of the patient’s level of renal function:
While the above may increase the probability of Gadolinium retention, cases of Gadolinium Toxicity (as evidenced by urinary Gadolinium levels) from a single contrast MRI were experienced by some participants in our original research; extravasation was not known to occur in any of these cases.
This describes some of what we have discovered about how one might become Gadolinium Toxic. To learn about how you might determine if you are suffering from Gadolinium Toxicity, continue reading the pages about Symptoms and Testing.
Establishing the Gadolinium Toxicity Connection
Symptoms are generally experienced at an acute level shortly after having a contrast MRI and at a chronic level for years following their last contrast MRI. Some people have the early acute symptoms that they can tie time-wise to their contrast MRI. Often they are very frightened, and any appeals to the medical professionals involved in the contrast ordering or administration process meet with denial or disbelief regarding the connection of their symptoms to the contrast agent, and certainly there is no supportive relief.
Others experience chronic symptoms that their doctors cannot explain and through research or testing they make the connection back to their contrast MRI. They, too, are concerned, but more from a viewpoint of “where is all this leading”. Many people experience both the early acute problems and the chronic effects. People at both ends of the spectrum want to know what they can do to cure their Gadolinium-related problems. More about that in our Treatment Possibilities section.
Symptoms of Gadolinium Toxicity
Some of the symptoms experienced fall outside normal descriptive terms for medical symptoms making it hard for patients to communicate to their doctors just what they are experiencing. For those symptoms, we will provide additional descriptive details as appropriate.
In rough order of frequency as reported in our Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs,
There is one symptom experienced by many that transcends several of the symptoms listed above. It is a sense of an electrified, vibrating, twitching feeling typically just under the skin that is sometimes localized and at other times a more overall feeling. Sometimes it feels like something is crawling around under the skin. This is a particularly alarming feeling when first experienced as it is unlike anything that the person has ever experienced and it is very difficult to explain to doctors.
Progression of Symptoms
Our research showed that there is very little difference between early symptoms and ongoing, chronic symptoms. But the experience of dealing with these symptoms and the impact it has on patients’ lives are often different.
Early Experience
Most people with Gadolinium Toxicity from contrast MRIs have symptoms within the first month after their contrast administration. For many, their symptoms start within a few days, and for some, within hours of being injected with the contrast agent. Usually the symptoms are intense, but for some the symptoms are more subtle. The experience can be frightening because the feelings are new and different; often, nothing is visible on the outside of the body. One’s mental or emotional state can be affected.
Generally, the intensity of the symptoms will subside over time, but the reduction is not necessarily uniform, with ups and downs. The frightened feeling also dissipates with time, and the symptoms may feel less intense as the mind and body get used to dealing with them. Reading some of the Viewpoints from people who have gone through this might be helpful and joining the MRI Gadolinium Support Group can provide interactive support.
Longer Term Chronic Experience
With time, symptoms may go away or significantly subside, but patients reported on in our Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs, have been dealing with their chronic symptoms for up to 5 years with no end in sight. With little medical attention, there are no known treatments to “cure” Gadolinium Toxicity (read more in Treatments). Symptom relief and coping methods will most often bring the patient into a state of being able to tolerate or simply accept their symptoms.
As anyone dealing with chronic conditions, patients experience ups and downs, and often try a variety of approaches to lessen the impact of their symptoms. For those whose symptoms do not go away, the intensity of the symptoms may increase over time. This would seem to indicate that the Gadolinium Toxicity is continuing to negatively impact their body.
It is much harder to describe the chronic experience because each of our bodies is different and our ability to cope is different. Since nothing has been published about patients with normal kidney function who developed NSF, we do not know if anyone with normal kidney function has died from their exposure to Gadolinium-Based Contrast Agents.
We are also not aware of anyone who has “cured” their Gadolinium Toxicity, although some on the MRI Gadolinium Support Group have reported improvement of some symptoms after trying various treatments. Some have been diagnosed with Small Fiber Sensory Neuropathy, thyroid abnormalities, adrenal fatigue, mast cell problems and other conditions. Often these diagnoses indicate some sort of atypical presentation of the associated symptoms. Since no related medical research has been published, we have no way of knowing whether there is a connection between the Gadolinium Toxicity and these conditions.
Our best advice is to hang in there and look for those actions that will help you deal with your symptoms and make sure your doctors know what you are experiencing. We would be happy to have you join the MRI Gadolinium Support Group to pass on your experience and learn from others.
Treatment Possibilities for Gadolinium Toxicity
Treatments. What everyone who is Gadolinium Toxic wants to know about. As you might expect, since Gadolinium Toxicity is not an established, accepted medical condition, there are no established, accepted medical treatments. However, by being connected with other Gadolinium Toxic people on the MRI-Gadolinium Toxicity Support group, we have learned about the treatment approaches that people have taken. We present some of those treatments below and you can also find more ideas in the Viewpoint section.
But first, we want to mention that the treatments tried with NSF patients are different from the ones that will be mentioned here. The majority of NSF patients were in kidney failure and on dialysis, and in most cases they presented with severe skin problems and joint contractures. To learn more about the treatments tried with NSF patients, we suggest that you visit the website of The International Center for Nephrogenic Systemic Fibrosis Research (ICNSFR) which is maintained by Shawn E. Cowper, MD.
Now to treatments tried by patients who are Gadolinium Toxic after contrast-enhanced MRI or MRA.
Treatments. What everyone who is Gadolinium Toxic wants to know about.
As you might expect, since Gadolinium Toxicity is not an established, accepted medical condition, there are no established, accepted medical treatments. However, by being connected with other Gadolinium Toxic people on the MRI-Gadolinium Toxicity Support group, we have learned about the treatment approaches that people have taken. We present some of those treatments below and you can also find more ideas in the Viewpoint section.
But first, we want to mention that the treatments tried with NSF patients are different from the ones that will be mentioned here. The majority of NSF patients were in kidney failure and on dialysis, and in most cases they presented with severe skin problems and joint contractures. To learn more about the treatments tried with NSF patients, we suggest that you visit the website of The International Center for Nephrogenic Systemic Fibrosis Research (ICNSFR) which is maintained by Shawn E. Cowper, MD.
Now to treatments tried by patients who are Gadolinium Toxic after contrast-enhanced MRI or MRA.
What is Gadolinium?
Gadolinium is a Rare Earth Element (REE) with paramagnetic properties. On the Periodic Table its symbol is Gd and its atomic number is 64.[1],[2] Gadolinium is one of 15 metallic chemical elements known as the Lanthanide Series. Lanthanides have been referred to as “bone seekers” because they tend to deposit in bone.[3], [4] Tests have confirmed that Gadolinium deposits in bone where it can remain for many years.[5],[6],[7],[8]
Gadolinium (Gd3+) has an ionic radius very similar to that of Calcium (Ca2+) which is why Gd3+ is so toxic in biological systems – Gd3+ can compete with Ca2+ in all biological systems that require Ca2+ for proper function and, in doing so, the trivalent Gd3+ ion binds with much higher affinity.[1]
Gadolinium is toxic to mammals and it has no known biological use in the human body. [9],[10],[11],[12],[13],[14],[15]
Gadolinium-Based Contrast Agents are used for MRI and MRA.Gadolinium has several specialized uses, but we will focus on its paramagnetic properties. In the early 1980s contrast agent developers determined that Gadolinium’s paramagnetic properties could be used to enhance images of abnormal tissue on Magnetic Resonance Imaging (MRI) scans.[16],[17]
Since free Gadolinium is toxic, the Gadolinium ion must be chelated or bound to a ligand (molecule) before it can be used as a contrast agent. The resulting complex is a Gadolinium-Based Contrast Agent or GBCA which is injected intravenously during a contrast-enhanced MRI.[18] GBCAs are also used for contrast-enhanced Magnetic Resonance Angiography (MRA). (Note that MRI and MRA are also performed without contrast.)
The intravenously administered GBCA should move rapidly through the body and then be excreted primarily via the kidneys before the toxic
Gadolinium ion and ligand can separate.[19] With normally functioning kidneys, most of the GBCA should be excreted in less than two hours after injection with the remainder being excreted over the next few days. However, impaired kidney function causes the GBCA to remain in the body for much longer periods of time which can result in the separation of the contrast agent and retention of the toxic Gadolinium ion.
In their 1984 report “Gadolinium-DTPA as a Contrast Agent in MRI: Initial Clinical Experience in 20 Patients”, Carr et al warned that “care should obviously be taken in patients with impaired renal and/or hepatic function where high in vivo concentrations of Gd-DTPA may occur for prolonged periods”.[20] Gd-DTPA, also known as Magnevist, was the first Gadolinium-Based Contrast Agent and it was approved by the FDA in 1988. (See Background on GBCAs for details.)
When severe skin problems first appeared in 1997 in a group of dialysis patients,[21] the connection was not made to that initial warning about the use of Gadolinium-Based Contrast Agents in renally-impaired patients. It wasn’t until 2006 that retained Gadolinium from GBCAs was confirmed as the probable cause.[22],[23]
When Gadolinium is retained in the body, it can have serious consequences – the most serious being an incurable and potentially life-threatening disease known as Nephrogenic Systemic Fibrosis or NSF which is thought to primarily affect patients with severely impaired kidney (renal) function. It is widely recognized by the medical community and government agencies that the toxicity of retained Gadolinium is the primary contributor to the development of NSF.
NSF was first thought to be a skin disease, but it was later learned that retained Gadolinium adversely affects all body systems by causing extensive fibrosis and calcification of connective tissue and internal organs. (See Background on NSF for details.)
Patients exposed to GBCAs are at risk of retaining Gadolinium.
The risks associated with the administration of Gadolinium-Based Contrast Agents to patients with severely impaired kidney function (eGFR <30) are well-documented and recognized by the medical community and FDA. Patients with normal kidney function are not thought to be at risk of retaining Gadolinium from GBCAs; however, published literature indicates that some Gadolinium from each dose of contrast may remain in the body of all patients exposed to GBCAs.[8], [24]
A 1991 article by Rocklage et al titled “Metal Ion Release from Paramagnetic Chelates: What is Tolerable?” reported that “minute amounts of chelated or unchelated metals are likely to remain in the body for an extended period and could possibly result in a toxic effect.” They also said it was “unlikely that MRI contrast agents would be administered repeatedly in patients”.[25] Unfortunately, many patients do have multiple MRI scans with contrast which can result in more Gadolinium remaining in the patient’s body.
Researchers have estimated that approximately 1% (15 mg) of the 1.5 grams of injected Gadolinium from each dose of contrast (0.1 mmol/kg body weight) may be released from the contrast agent and deposited in the bones of GBCA exposed patients including those with normal kidney function.[26]
The long-term and cumulative effects of retained Gadolinium are unknown as are the additive effects of the double or triple doses often used for contrast MRAs.
Besides severely impaired kidney function, there are other factors that can increase the risk of retaining Gadolinium. Those risk factors include acidosis, transient acute kidney injury (AKI), recent surgery, inflammatory events, abnormal vascularity, compromised blood-brain barrier, cumulative dosage, and transmetallation.[27],[28],[29],[30] Other than kidney impairment, researchers have found that transmetallation presents the greatest potential risk for the release of the toxic metal ion from the chelate.[25], [31] Transmetallation is a potential risk for every GBCA exposed patient. (See Risk Factors for more details.)
Gadolinium can deposit in brain tissue.
MRIs with contrast are frequently performed when the brain is being imaged. The brain is protected from potentially harmful compounds in the blood by the semi-permeable Blood-Brain Barrier (BBB). The intact BBB protects the brain from damage, whereas the dysfunctioning BBB allows influx of normally excluded hydrophilic molecules into the brain tissue.[32]
A brain tumor or lesion causes a disruption or break in the BBB. That “break” gives the Gadolinium-Based Contrast Agent access to the brain – regardless of the patient’s level of renal function at the time of his or her contrast procedure. So...TBI survivors and Stroke survivors who undergo multiple MRI's with contrast are at risk of Gadolinium Toxicity...Yep. I'm in that group...TBI Survivor.
GBCA product labeling indicates that Gadolinium-Based Contrast Agents “do not cross an intact blood-brain barrier”; however, “disruption of the blood-brain barrier” or “abnormal vascularity” allows accumulation in lesions such as neoplasms (tumors), abscesses, and subacute infarcts.[33],[34],[35],[36],[37],[38],[39],[40] That is why Gadolinium is deposited in brain tumors and brain lesions such as those seen in Multiple Sclerosis. The abnormal brain tissue then becomes more clearly defined or enhanced on magnetic resonance (MR) images.[41] Gadolinium should not remain in the body; however, the literature indicates that Gadolinium retention has occurred.
A 1995 case report by Martinez noted persistent Gadolinium retention in an extra-axial brain stem lesion of a patient with Erdheim-Chester Disease. Gadolinium retention was confirmed by comparing precontrast and postcontrast images from two MRIs performed 23 days apart.[42] A 1989 case report of Erdheim-Chester disease reported persistent enhancement of intracerebral lesions 8 days after injection with Gd-DTPA. Chemical analysis of the biopsy specimen revealed a high concentration of Gadolinium.[43]
A 2009 study by Fulciniti et al found that a Gadolinium-containing contrast agent promoted Multiple Myeloma (MM) cell growth. Autopsies of 8 MM patients with repeated exposure to the agent found “massive amounts of Gadolinium accumulation in tissues regardless of their renal function”.[44]
A 2010 study by Xia et al found insoluble deposits containing Gadolinium in 7 brain tumor biopsies from 5 patients whose eGFRs were above 53 ml/min, confirming that brain tumors alter the Blood-Brain Barrier which allows the injected GBCA to deposit in brain tissue, even in patients without severe kidney problems.[45]
A 2013 study by Kanda et al found that abnormally high signal intensity detected in two regions of the brain on unenhanced T1-weighted images was related to the number of previous Gadolinium-based contrast enhanced MRIs that had been undergone by the population of patients with normal renal function.[46]
Patients with Multiple Sclerosis (MS) routinely have MRIs with contrast to monitor disease activity and response to treatment.
Gadolinium will enhance “active” MS lesions due to a breakdown in the Blood-Brain Barrier.[47] Serial contrast MRIs have shown that active lesions generally enhance for a period of 1 to 2 months on average.[48],[49]The enhancing lesion evolves to a chronic T2 hyperintense lesion, which is the non-specific ‘footprint’ of the prior inflammatory event.[50] It has been speculated “that the initial enhancing-inflammatory lesion events in the brain, place into motion, at an early stage, the processes that ultimately lead to cerebral atrophy, and these processes may include early axonal injury”.[50]
While the role of enhancing-inflammatory lesions in the development of cerebral atrophy in MS is unclear, studies have demonstrated that cerebral atrophy paralleled that of contrast enhancing lesion accumulation.[51],[52] As noted by Simon, atrophy, particularly that resulting from volume loss around the third ventricle, appears to be predicted by the presence of enhancing lesions at baseline.[50]
Patients without enhancing lesions at baseline showed no increment (increase) in third ventricle width, while the enhancing group showed significant increments in atrophy.[50] The enhancement is the result of deposited Gadolinium; however, the literature does not indicate whether or not Gadolinium deposition or retention leads to brain atrophy.
Retention of Gadolinium from contrast MRIs has been confirmed in a patient with MS. Results of provoked urine testing for toxic metals after administration of the chelating agent EDTA showed high levels of Gadolinium.[53] The chelating agent can extract elements from tissue in much higher quantity than would normally be excreted in urine. Removal of Gadolinium and other metals by chelation therapy was reported to definitively improve the patient’s MS symptoms.
While the presence of a brain tumor or lesion alters the Blood-Brain Barrier, there are other ways that the BBB might be crossed or temporarily altered, such as the following:
- Lack of a BBB on the Optic Nerve Head,[54],[55] circumventricular organs (CVOs)[56] and Chorid Plexuses [57],[58]
- Having Type II Diabetes or white matter hypersensitivity like hypertension [59]
- Diffusion into the vitreous and aqueous humors of the ocular globes, perivascular spaces, and ventricles of the brain[60]
- Diffusion into the subarachnoid[61],[62],[63] and subdural spaces[64]
- Effects of Electromagnetic Pulse (EMP)[65],[66],[67],[68] and Electromagnetic Fields (EMF)[69],[70],[71]
Note that findings of delayed and hyperintense enhancement of Cerebrospinal Fluid (CSF) have been reported in patients with normal renal function.[62],[63],[72],[73],[74]
Here are other important facts about Gadolinium and GBCAs.
- Gadolinium is neurotoxic. [75],[76],[77],[78] It inhibits mitochondrial function and induces oxidative stress.[79],[80]
- Gadolinium-Based Contrast Agents can be nephrotoxic. [81],[82],[83],[84],[85],[86],[87]
- Residual Gadolinium from GBCAs has been found in bone and other tissue of study animals that did not have NSF-like skin lesions.[88],[89],[90],[91],[92]
- A 2004 study by Gibby confirmed deposition of Gadolinium in bone tissue from patients with normal renal function 4 days after exposure to a GBCA. [93]
- A 2008 study by Abraham et al presented findings that indicate deposited Gadolinium (Gd) may be mobilized over time from bone stores. – “regardless of renal function at present”. [94]
- A 2009 study by Darrah et al found Gadolinium in hip bone removed from a patient 8 years after her last contrast MRI, confirming Gadolinium deposits in bone where it can remain for many years.[8]
- A 2011 Mayo Clinic Study by Christensen et al found detectable concentrations of Gadolinium in fresh tissue specimens taken from two control subjects with normal renal function. Both patients had previous GBCA exposure, one 8 months and the other 16 months before their biopsy.[95]
- The release of Gadolinium from all linear Gd3+ complexes in human serum from healthy volunteers was several orders of magnitude greater than predicted by conditional stability constants.[96]
- Studies have shown that GBCAs affect blood and tissue of subjects with normal kidney function. [97],[98],[99],[100],[101],[102]
- All GBCAs and Gadolinium Chloride (GdCl3+) have been found to stimulate fibroblast proliferation in tissue taken from healthy subjects. (Fibroblasts play a role in the production of connective tissue and fibrosis.) [99],[103],[104]
- Macrocyclic agents are thought to be more stable and thereby safer to use; however, even they have been found to deposit in tissue.[88],[105],[106] There are now confirmed cases of NSF caused by macrocyclic agents as well. [107],[108]
- Retained Gadolinium affects more than the skin. Autopsies of deceased NSF patients have found extensive multiorgan fibrosis and calcification, as well as vascular and extracellular deposits of Gadolinium.[109] Areas affected include: liver, lungs, intestinal wall (ileum), kidney, lymph node, skeletal muscle, diaphragm, mitral valve, aortic arch, great vessels of the heart, cardiac conduction system, left ventricle and septum, blood vessels, atrial myocardium, lesser pelvis, testes, adrenal glands, pancreas, colon, thyroid, gastrointestinal tract including esophagus and stomach, eye, brain parenchyma, subarachnoid space, dura mater including that surrounding the spinal cord.[110],[111],[112],[113],[114],[115],[116],[117],[118],[119],[120],[121]
- Patients with normal kidney function are excreting levels of Gadolinium that are well above Mayo Laboratories Reference Range (>0.4 mcg/specimen) as long as several years after their last contrast-enhanced MRI or MRA. (See Self-Study of Retained Gadolinium and Appendix 1 of Symptom Survey Report.)
- Results of our Symptom Survey Report show high levels of commonality in participants’ chronic symptoms of Gadolinium Toxicity. The symptoms are similar to those of NSF patients (See Appendix 2 of Symptom Survey Report.)
The FDA and medical industry directly involved with NSF and GBCAs are aware of these facts. However, clinicians have been told that patients with normal kidney function do not retain Gadolinium and are therefore not at risk from a contrast-enhanced MRI or MRA. Because of that, Gadolinium-related health problems may be greatly under reported.
Gadolinium and MRIs: A Common, Risky Connection
When patients get MRIs, they most commonly hear that they will be injected with a “dye” that will help the hospital staff read the results. What they are not told is that in about 30% of cases, this “dye” is actually gadolinium, an extremely poisonous metal.
The medical community thinks that it is pretty harmless, and that it leaves the body right after an MRI is done. As we see from Gena’s horrible experience, that is not always the case.
The internal FDA documents show that many scientists have known for over a decade that this issue exists, yet nothing has been done to protect millions of people who get MRIs every year.
(About 30 million Americans get an MRI every year; that’s one in every 10 people. Three countries that administer even more MRIs than the U.S. are Japan, Germany and Turkey)
In 2006, the FDA recognized that there is a strong link between gadolinium and nephrogenic systemic fibrosis (NSF), a deadly condition that leads to thickening of organs and skin.
In 2007, they had to add a warning to gadolinium stating that it can put patients who have weak kidneys at risk as their bodies might not be able to eliminate this toxin from their bodies.
The first problem is that many people and their doctors have no idea that they have weak kidneys.
The second problem is this is not the case with every patient.
Sharon Williams’s health changed for the worst after her 5th MRI. (founder of www.GadoliniumToxicity.com)
After Gena, Full Measure host interviewed Sharon Williams, a patient advocate for gadolinium poisoning and one of the two founders of GadoliniumToxicity.com
Sharon had five MRIs in her life, and after each one her health deteriorated more and more. However, she has zero kidneys problems, which shows that gadolinium can be toxic to people with functioning kidneys as well.
A test has revealed that she had this heavy metal stored in her thyroid gland and brain. After MRIs she developed a rash and other skin problems, which made her lose hair on her legs. She had symptoms in her abdomen, her ribs, and her head. She was in pain. She had muscle spasms, high blood pressure, and poor cognitive function.
After realizing the connection between her health struggles and gadolinium, Sharon created GadoliniumToxicity.com together with Hubbs Grimm to educate patients. She has collected 500 research articles about gadolinium and its toxicity, and now fights for appropriate treatment for all who are affected by this heavy metal.
Modern medical treatment for gadolinium poisoning is highly expensive and is difficult to find. The Norris family spent over $2 million on Gena’s treatment. This is not something many families can afford.
“We’ve been blessed enough to be able to afford the alternative or the integrative treatments and modalities and the medicines that are out there. There are millions of people that, that don’t have that, okay, and because insurance doesn’t pay,” Gena said.
Pharmaceutical Companies’ Grip on Gadolinium
The FDA has so far done nothing about the gadolinium issue. But the information is slowly penetrating the public and affecting government policy. Last year in Europe, a government health committee made a recommendation that gadolinium should be suspended from use, even as it is still routinely administered to patients here in the U.S.
One of the major companies that makes it, General Electric’s (GE) health care division, asked officials to reconsider the ban. General Electric, pharmaceutical company Bayer, and the FDA all claim that “the risk benefit profile [for gadolinium products] remains favorable.”
Instead of banning this chemical, the FDA approved it for use for infants and babies under two years old. It plans to hold a public meeting to discuss the gadolinium issue in the future.
By means of a symptom survey of 17 people with high urine levels of Gadolinium, we have provided a comprehensive review of this topic in Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs, which we encourage you to read. On this page, we will provide some high-level information from that paper as well as other observations we have gathered from MRI Gadolinium Support Group.
Getting Gadolinium Toxicity from Contrast MRIs
When undergoing an MRI with contrast, a Gadolinium-Based Contrast Agent (GBCA) is injected into your vein. All of the people who order and administer the GBCA believe that it is totally safe for people with normally functioning kidneys and that it will be gone from your body within a few days via your urinary tract.
Many, many people have received contrast MRIs with no adverse effects that they have identified as related to or caused by the contrast agent. But we, ourselves, and others active in the Support Group and participants in the original research that we conducted, feel strongly that we have been adversely affected by retained Gadolinium from the contrast administration.
Although there has been no published direct measurement of retained Gadolinium, it has been estimated that 1% of the injected Gadolinium from each dose of contrast is retained in the body. Our research supports the idea that Gadolinium is retained as evidenced by urine testing for Gadolinium.
In our Study of Retained Gadolinium from Contrast MRIs report, we document urinary Gadolinium levels in 24-hour collections that show Gadolinium retention well beyond three days.
Based on much of the published medical research on GBCAs and NSF, the basic problem appears to be that the toxic Gadolinium ion separates from the chelate (ligand) to which it was bound in the manufacturing process in order to reduce the toxicity of the metal ion; it is postulated that the longer the GBCA remains in the body, the greater the likelihood of this separation. This is supported by the predominance of NSF patients having impaired renal function, which caused the GBCA to remain in the body longer.
Other than renal insufficiency, no one knows all the co-factors that may cause one person to retain or react to Gadolinium while another person does not. However, the following may increase the risk of Gadolinium Toxicity regardless of the patient’s level of renal function:
- Multiple contrast MRIs – Both of the website authors, and others we have been in contact with, have had more than a single contrast MRI. If the body retains 1% of the Gadolinium from each dose of contrast, it seems rather straightforward that multiple contrast MRI could increase the risk.
- Higher than normal GBCA dosage or multiple contrast MRIs within a short period of time – We have no quantifiable information, but here also, both these scenarios would result in higher levels of GBCAs in the body for a longer period of time, increasing the probability of separation of the toxic Gadolinium ion from the ligand.
- Extravasation during the intravenous infusion process – Extravasation is the accidental injection of the contrast agent into surrounding soft tissue rather than directly into the vein. Besides causing damage to the surrounding tissue, the extravasated portion of the GBCA dose would not be readily available for excretion by the kidneys which could result in increased Gadolinium retention.
- Transmetallation or dissociation of the GBCA – Transmetallation is the displacement of the Gadolinium ion from its ligand by other metals in the body such as zinc, copper, calcium, and iron. The metals can work simultaneously to destabilize the GBCA complex and lead to its dissociation which results in Gadolinium remaining in the body.
While the above may increase the probability of Gadolinium retention, cases of Gadolinium Toxicity (as evidenced by urinary Gadolinium levels) from a single contrast MRI were experienced by some participants in our original research; extravasation was not known to occur in any of these cases.
This describes some of what we have discovered about how one might become Gadolinium Toxic. To learn about how you might determine if you are suffering from Gadolinium Toxicity, continue reading the pages about Symptoms and Testing.
Establishing the Gadolinium Toxicity Connection
Symptoms are generally experienced at an acute level shortly after having a contrast MRI and at a chronic level for years following their last contrast MRI. Some people have the early acute symptoms that they can tie time-wise to their contrast MRI. Often they are very frightened, and any appeals to the medical professionals involved in the contrast ordering or administration process meet with denial or disbelief regarding the connection of their symptoms to the contrast agent, and certainly there is no supportive relief.
Others experience chronic symptoms that their doctors cannot explain and through research or testing they make the connection back to their contrast MRI. They, too, are concerned, but more from a viewpoint of “where is all this leading”. Many people experience both the early acute problems and the chronic effects. People at both ends of the spectrum want to know what they can do to cure their Gadolinium-related problems. More about that in our Treatment Possibilities section.
Symptoms of Gadolinium Toxicity
Some of the symptoms experienced fall outside normal descriptive terms for medical symptoms making it hard for patients to communicate to their doctors just what they are experiencing. For those symptoms, we will provide additional descriptive details as appropriate.
In rough order of frequency as reported in our Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs,
- Pain – aching; burning, tingling, and/or prickling pain (paresthesia); deep bone pain. Typically in extremities or joints, and sometimes in the location where the MRI occurred, like the head.
- Dermal changes – like tight skin, lesions, hyperpigmentation. Most often in extremities.
- Muscle issues – twitching – small, local, rapid contractions and weakness
- Ocular problems – worsening vision, dry eyes, bloodshot eyes
- Cognitive symptoms
- Ear, nose and throat – tinnitus, swallowing, and voice problems
- Low body temperature
- Hair loss
- Itchy skin
- Balance problems
- Swelling of extremities (edema)
There is one symptom experienced by many that transcends several of the symptoms listed above. It is a sense of an electrified, vibrating, twitching feeling typically just under the skin that is sometimes localized and at other times a more overall feeling. Sometimes it feels like something is crawling around under the skin. This is a particularly alarming feeling when first experienced as it is unlike anything that the person has ever experienced and it is very difficult to explain to doctors.
Progression of Symptoms
Our research showed that there is very little difference between early symptoms and ongoing, chronic symptoms. But the experience of dealing with these symptoms and the impact it has on patients’ lives are often different.
Early Experience
Most people with Gadolinium Toxicity from contrast MRIs have symptoms within the first month after their contrast administration. For many, their symptoms start within a few days, and for some, within hours of being injected with the contrast agent. Usually the symptoms are intense, but for some the symptoms are more subtle. The experience can be frightening because the feelings are new and different; often, nothing is visible on the outside of the body. One’s mental or emotional state can be affected.
Generally, the intensity of the symptoms will subside over time, but the reduction is not necessarily uniform, with ups and downs. The frightened feeling also dissipates with time, and the symptoms may feel less intense as the mind and body get used to dealing with them. Reading some of the Viewpoints from people who have gone through this might be helpful and joining the MRI Gadolinium Support Group can provide interactive support.
Longer Term Chronic Experience
With time, symptoms may go away or significantly subside, but patients reported on in our Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs, have been dealing with their chronic symptoms for up to 5 years with no end in sight. With little medical attention, there are no known treatments to “cure” Gadolinium Toxicity (read more in Treatments). Symptom relief and coping methods will most often bring the patient into a state of being able to tolerate or simply accept their symptoms.
As anyone dealing with chronic conditions, patients experience ups and downs, and often try a variety of approaches to lessen the impact of their symptoms. For those whose symptoms do not go away, the intensity of the symptoms may increase over time. This would seem to indicate that the Gadolinium Toxicity is continuing to negatively impact their body.
It is much harder to describe the chronic experience because each of our bodies is different and our ability to cope is different. Since nothing has been published about patients with normal kidney function who developed NSF, we do not know if anyone with normal kidney function has died from their exposure to Gadolinium-Based Contrast Agents.
We are also not aware of anyone who has “cured” their Gadolinium Toxicity, although some on the MRI Gadolinium Support Group have reported improvement of some symptoms after trying various treatments. Some have been diagnosed with Small Fiber Sensory Neuropathy, thyroid abnormalities, adrenal fatigue, mast cell problems and other conditions. Often these diagnoses indicate some sort of atypical presentation of the associated symptoms. Since no related medical research has been published, we have no way of knowing whether there is a connection between the Gadolinium Toxicity and these conditions.
Our best advice is to hang in there and look for those actions that will help you deal with your symptoms and make sure your doctors know what you are experiencing. We would be happy to have you join the MRI Gadolinium Support Group to pass on your experience and learn from others.
Treatment Possibilities for Gadolinium Toxicity
Treatments. What everyone who is Gadolinium Toxic wants to know about. As you might expect, since Gadolinium Toxicity is not an established, accepted medical condition, there are no established, accepted medical treatments. However, by being connected with other Gadolinium Toxic people on the MRI-Gadolinium Toxicity Support group, we have learned about the treatment approaches that people have taken. We present some of those treatments below and you can also find more ideas in the Viewpoint section.
But first, we want to mention that the treatments tried with NSF patients are different from the ones that will be mentioned here. The majority of NSF patients were in kidney failure and on dialysis, and in most cases they presented with severe skin problems and joint contractures. To learn more about the treatments tried with NSF patients, we suggest that you visit the website of The International Center for Nephrogenic Systemic Fibrosis Research (ICNSFR) which is maintained by Shawn E. Cowper, MD.
Now to treatments tried by patients who are Gadolinium Toxic after contrast-enhanced MRI or MRA.
Treatments. What everyone who is Gadolinium Toxic wants to know about.
As you might expect, since Gadolinium Toxicity is not an established, accepted medical condition, there are no established, accepted medical treatments. However, by being connected with other Gadolinium Toxic people on the MRI-Gadolinium Toxicity Support group, we have learned about the treatment approaches that people have taken. We present some of those treatments below and you can also find more ideas in the Viewpoint section.
But first, we want to mention that the treatments tried with NSF patients are different from the ones that will be mentioned here. The majority of NSF patients were in kidney failure and on dialysis, and in most cases they presented with severe skin problems and joint contractures. To learn more about the treatments tried with NSF patients, we suggest that you visit the website of The International Center for Nephrogenic Systemic Fibrosis Research (ICNSFR) which is maintained by Shawn E. Cowper, MD.
Now to treatments tried by patients who are Gadolinium Toxic after contrast-enhanced MRI or MRA.
The ideas presented below are purely anecdotal treatment approaches that members of our support group have tried. While some of the treatment approaches have been tried by multiple people, no controlled trials of any treatment mentioned have been conducted. Nothing that follows is medical advice. You should consult a medical professional before starting any treatment or taking any supplements. There are no FDA approved over-the-counter (OTC) chelation products.
Chelation
The most obvious treatment is to try to remove the Gadolinium from your body with Chelation. Chelation therapy is a medical process that involves administering chelating agents that will bind to the metal ions to form a more chemically stable compound that can be safely excreted from the body, typically through the kidneys. Most Chelation is done without FDA approval of the chelating process.
Although ethylenediaminetetraacetic acid (EDTA), and specifically Calcium EDTA is the agent we have seen used most often in doctor-controlled IV Chelation to remove Gadolinium, it is only approved by the FDA for removal of lead. Nonetheless, Chelation therapy is practiced every day by doctors providing alternative or integrative medical services, and is best done under their supervision because the chelating agent can also remove important nutrients like calcium and zinc from the body.
While Chelation might seem obvious, we are not aware of anyone who has removed all of his or her retained Gadolinium (as determined by urine testing) or is totally free of the symptoms that they attribute to Gadolinium. Some have reported improvement in the severity of their symptoms while others have reported feeling worse while doing Chelation. Without a controlled trial, there is no way to know for sure that these improvements or worsening of symptoms are the result of Chelation or are just the natural course of symptom progression for those individuals.
Nonetheless, we know that everyone wants to know how they can remove the retained Gadolinium from their body. Below are different methods that people have reported using.
IV Chelation
The strongest approach is with intravenous (IV) infusion of a chelating agent(s) by a doctor. Typically the doctor will conduct a provoked urine test to determine the body burden of various toxic substances. He or she will then determine the appropriate chelating agent(s) and recommend a course of treatment. Treatments are usually done between one and three times a week for a total of more than 20 sessions – we know people who have done more than 50 IV chelation treatments. There is sometimes additional urine testing to monitor progress, and there are sometimes breaks during the course of treatments.
These IV chelation treatments performed by a doctor may range from $100 to $250 per session and are not normally covered by insurance.
However, after working with their doctor and their insurance company, some people have been able to have their IV Chelation covered, especially if high levels of other metals like mercury or lead were found. One of the biggest advantages of doing IV Chelation is that the patient is working with a doctor familiar with the benefits and risks of IV Chelation so that the best possible course of treatment is followed.
Oral Chelation
Oral chelation can either be done with a chelating agent in pill or capsule form, or it can be done with herbs and other foods. Chelating with a pill or capsule is usually done under the watchful eye of a doctor who can advise you on additional supplements that are needed to counteract the removal of good minerals by the chelating agent as it removes the toxic heavy metals.
Some patients looking for a more natural way to remove the Gadolinium have studied the chelating properties of foods and have focused on foods that they are hopeful will help. Herbs such as cilantro and garlic, pectin and chlorella, and supplements such as Alpha Lipoic Acid (ALA) and N-Acetyl-Cysteine (NAC) have some chelating properties and may be worthy of additional research.
Sublingual Powders and Suppositories
Sublingual is different from Oral Chelation because the EDTA is placed under the tongue and absorbed directly into the body rather than going through the digestive process. With rectal suppositories, EDTA is absorbed into the highly absorbable rectal mucosa. Both of these are also best done under a doctor’s care, although the products may be available for purchase online or in health food stores.
Regardless of the method used for Chelation, it is best done under a doctor’s care, or the patient must be committed to lengthy research and self-monitoring of important mineral levels. There is additional information on Chelation available in the Viewpoints section, but the information presented is someone’s personal viewpoint on chelation and it might not agree with the views of others regarding chelation. We encourage you to do your own research, confer with your doctor, and reach a decision that is best for you.
Skin Therapies
This is a somewhat artificial grouping of several different approaches that all happen to involve the skin. The skin is significant since it is the primary focus of the workup to determine if someone has NSF. The skin has also been referred to as the “third kidney” because the skin can also help the body get rid of toxins.
Saunas
Several people in the believe that either FAR Infrared or Near Infrared saunas can help keep the skin healthy while aiding the skin in ridding the body of toxins. We will not try to enter the debate on whether FAR or NEAR Infrared saunas are best. You can learn more from members of the Support Group or do your own research.
Epsom salt baths
Another idea that may have merit is Epsom salt baths because of their ability to get the salts, particularly magnesium into the body and the muscles. The anecdotal evidence is that they may reduce some of the pain associated with Gadolinium Toxicity.
Symptom Relief
Symptom relief is a very broad topic and one that clearly should be a matter of discussion between the patient and their doctor. In our Survey of Chronic Symptoms of Gadolinium Toxicity, 100% of the participants reported Pain as one of their top symptoms. Pain can reorient your lifestyle, and cause you both mental and physical distress. Our only advice is to consider symptom relief carefully, and do not try to be the hero who says “I can tough it out”. Both of the website authors take prescribed medications for pain, and although neither is totally pain free, both are able to live a fairly normal life as a result of living with less pain on a daily basis.
Healthy Eating (and supplements)
Healthy eating and supplements is also a very broad topic. Depending on whether the patient sees a regular Primary Care physician, a Wellness doctor or a Naturopathic doctor, he or she may be presented with different recommendations on ways to keep their nerves healthy, invigorate their muscles, reduce pain, or address their particular symptoms. Many of the Support Group members have made Healthy Bodies part of their treatment regimen.
As with Testing, we wish we had a better situation to report on for Treatments, but it is not the case. Until the medical community recognizes Gadolinium Toxicity as a medical condition, we will have to rely on ourselves, our caregivers, and each other to learn about possible treatment approaches.
Testing for Gadolinium Toxicity
Since there are no established testing methodologies, none of the testing methods described below should be taken as the final word. Since Gadolinium tends to deposit in bone and not remain in circulation, it could be that you are Gadolinium Toxic with negative test results.
Urine Testing
Although Urine Testing may not be definitive in determining if you are Gadolinium Toxic, we believe that consistent, high urine test results for Gadolinium are a positive indicator for Gadolinium Toxicity. As described below, it can easily be repeated periodically for progress monitoring.
A word of caution – Having elevated urine levels of Gadolinium will not get you a Gadolinium-related diagnosis, it will only provide proof that you retained Gadolinium. And having a lower level of Gadolinium in your urine long after your contrast MRI does not mean that you did not have a much higher level at earlier time points.
For those who believe they are suffering from Gadolinium Toxicity, a 24-hour unprovoked urine test can provide some insight.
If you have not done so yet, we recommend that you read the Study of Retained Gadolinium from Contrast MRIs and the Appendix 1 of the Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs to understand the urine testing results that we have documented.
Before providing information about having your urine tested for Gadolinium, a few words about types of testing, urine collection, and results reporting are appropriate.
First, a test can be either Provoked or Unprovoked as described in our Study of Retained Gadolinium from Contrast MRIs.
An Unprovoked test will tell you the amount of Gadolinium that is normally being excreted. We recommend this as the first test you have done to establish a baseline measurement. Later you can have other Unprovoked tests to see how your level has come down. Your unprovoked levels can be compared with the results of others as reported in the Study of Retained Gadolinium from Contrast MRIs. Results can also be compared with the reference range established by Mayo Labs for their 24-hour Gadolinium Urine test (0.0-0.4 mcg Gd/specimen). Other testing labs may use lower ranges, but we typically refer to the Mayo Labs range in our discussions.
A Provoked test is done by collecting the urine sample after taking a provoking agent (or having an IV with a chelating formula) to attempt to ‘draw out’ additional toxic metals from tissues that would not have come out with an Unprovoked test. While a provoked test may identify a higher ‘body burden’ of Gadolinium, these tests cannot easily be compared with results from other individuals because the specific provoking or chelating formula used by each doctor will significantly affect the test results. There are no established reference ranges for Provoked testing other than those established by the testing companies.
Second, a test can be done for differing lengths of time.
A Random Collection is from a single urination; the results will have greater variability due to factors such as time of day, recent food or liquid intake, etc.
A 24-hour collection provides the most consistent results as it reduces the factors that can lead to variability in a single collection.
A 6-hour collection is often done for a provoked test since the time-effectiveness of the provoking agents is generally a couple hours, and the results would be lower if the collection was done for 24 hours.
Third, the units of measurement used in the test must always be understood.
Test results are normally expressed as either mcg Gd/sample (micrograms of Gadolinium per urine sampling period) or mcg Gd/g Creatinine .
Both of these measures will be reasonably consistent for an individual. However these two different units cannot be compared with each other. Comparing a result of 0.6 mcg Gd/24-hours with a result of 0.9 mcg Gd/g Creatinine would be like comparing the statement “I used 60 gallons of gas on my 24-hour trip to Boston” with “I got 23 miles per gallon while on my trip to Boston”. We do not know which car gets greater fuel efficiency.
For these reasons, we recommend an unprovoked 24-hour collection if you want to compare the results with others or track your levels over an extended time period.
Getting a random or 24-hour unprovoked test for Gadolinium is not exactly straightforward, but it can be done. Your doctor can order the 24-hour test from Mayo Clinic Medical Laboratories through testing agencies like Quest Diagnostics and others. But he or she will likely not be familiar with this test as it is not regularly prescribed. You may need to request the test.
A wellness doctor or a naturopathic doctor will be the best source for having urine testing conducted by Genova Diagnostics or Doctor’s Data independent clinical laboratories. These labs do their testing for Gadolinium as part of a toxic metals panel that is typically used by the doctor to determine if Chelation might benefit the patient. More about Chelation can be found in the Treatmentssubsection.
Since many doctors performing Chelation routinely only do 6-hour provoked urine testing, we want to repeat the suggestion that you periodically have a 24-hour unprovoked urine test to enable tracking of your urine Gadolinium when not under the influence of a provoking or chelating agent. You might want to check the Chelation Category in our Viewpoints section where individual Chelation results are discussed.
The Genova Diagnostics urine test for Gadolinium is also available through several online services. The collection and testing process is exactly the same as if done through a local doctor except that the report is sent directly to the patient. Genova Diagnostics has two test panels that include the Gadolinium test. You should get either the Toxic Elements Clearance Profile or the Comprehensive Urine Element Panel.
Make sure you check out the online sample reports that also include interpretive comments for any tests that are out of range. Please note that we have no connection of any sort with Genova Diagnostics and we do not make any statement about their validity for any particular purpose. One of the authors has used Genova Diagnostics many times, but we only provide these links as an information service. A patient cannot purchase these tests directly from Genova Diagnostics.
The online services we have found where these tests can be purchased include Health Remedies, Forrest Health, and likely others. If you are outside the United States you may need to find a local testing source. For example, Healthscope Pathology in Australia can arrange for testing through Genova Diagnostics.
Blood Testing
Blood Testing (also called Serum Testing) for Gadolinium is also available through Mayo Clinic Medical Laboratories. However, the plasma half-life of Gadolinium is approximately 90 minutes. Mayo states that elevated Gadolinium in serum drawn more than four days after GBCA administration is not typical of most patients with normal renal function. Our experience is that blood tests often report undetectable levels when urine tests indicate elevated levels of Gadolinium. We will not cover blood testing in any additional detail.
Biopsy Testing
Biopsy Testing is a more complex topic including two different types of tests – dermal biopsies for histological features similar to those found in NSF patients, and testing of tissue for the presence of Gadolinium.
We want to be clear that in our view, Gadolinium Toxicity is not the same as NSF, but likely shares some similarities with NSF due to retention of Gadolinium. Nonetheless, we understand that some patients will want to be tested for NSF.
There is no biopsy or laboratory test that can be used as a gold standard to diagnose NSF. However, the current clinicopathological criteria focus heavily on clinical and histologic findings that involve the skin and underlying tissues. Finding evidence of Gadolinium in tissue is not a criteria used in the diagnosis of NSF.
For the most part, the histopathological criteria for NSF are based on what was found in patients with severe renal impairment after exposure to Gadolinium-based Contrast Agents. In other words, it is based on what was seen in patients who likely retained large amounts of toxic Gadolinium due to their poor renal clearance of the contrast agent.
Most dermatopathology labs can test dermal biopsies for the histopathological features seen in NSF; however, most patients with normal kidney function do not present with the same, severe skin manifestations after Gadolinium retention. If you have skin changes and plan to have dermal tissue tested for NSF, it should be taken from an area with skin changes that are similar to what has been seen with NSF.
Before you have a biopsy, be sure your dermatologist and his or her lab are familiar with the biopsy specimen requirements and the histopathological evaluation that is required to make an NSF diagnosis. It is very important that the biopsy be deep enough, have enough volume, and be taken from affected skin.
If you have NSF-like dermal changes, you can also have a biopsy specimen tested for the presence of Gadolinium at Mayo Medical Laboratories. Note that providing evidence of Gadolinium in your dermal tissue is not required to get an NSF diagnosis. Mayo Clinic’s testing has been set up in relation to people contracting NSF (Nephrogenic Systemic Fibrosis). The most important thing to note is that even in patients diagnosed with NSF, higher concentrations of Gadolinium are usually found in tissue taken from an “affected” area rather than in tissue taken from an
“unaffected” area. Most of the people who have been participants in our research do not have skin changes consistent with what would be considered NSF-like “affected” tissue.
Even though many participants in our Symptom Survey identified Dermal Issues as one of their symptoms, we are not aware of any positive dermal biopsy results for these individuals – either for the histological features of NSF or for the presence of Gadolinium.
During the course of your medical care, a biopsy or removal of other body tissue might be medically warranted. That tissue can also be tested for the presence of Gadolinium; however, to our knowledge, that testing is not available for purchase at any lab. Testing would have to be arranged with a researcher who may be willing to test tissue specimens for evidence of Gadolinium.
There is no guarantee that anyone will be willing to do the testing, and any cost involved might not be covered by insurance. Contact us if you would like additional information on searching for researchers who might be willing to help you. Also note that even if Gadolinium were discovered in tissue, the medical community has no reference levels or other ways to evaluate what the presence of Gadolinium might mean.
Many of the symptoms we describe as being related to Gadolinium Toxicity are not visible on the skin or related to the skin. Therefore the dermal test is not relevant for things like muscle pain, paresthesias, cognitive issues, or ocular and ENT symptoms. The current Gadolinium-related diagnostic criteria are based solely on what has been seen in patients with severe renal impairment who developed NSF, and that is based primarily on dermal changes and joint contractures. There are no tests to evaluate the effects of Gadolinium Toxicity on internal organs and body tissues.
We would like the Testing story to be a better one, but it is not. We should all continue our advocacy efforts to drive for recognition of Gadolinium Toxicity and the development of relevant diagnostic tests.
Editorial comment:
Note the various findings in patients with normal kidney function mentioned throughout this Background on Gadolinium. It would seem that these findings might explain why patients with normal kidney function (meaning eGFR >60) are presenting clinically with various symptoms of Gadolinium Toxicity after a contrast MRI or MRA. The severity of each patient’s symptoms likely depends on the total amount of Gadolinium that he or she retained from the intravenously administered Gadolinium-based Contrast Agent.
NSF may be the most severe manifestation of Gadolinium Toxicity, but there is no logical reason to think it is the only one. We believe that Gadolinium Toxicity is a “disease of degrees”, all of which are potentially serious and life-changing.
Have you been affected by retained Gadolinium from a contrast MRI?
Anyone who has unexplained symptoms that you believe were caused by retained Gadolinium from a contrast MRI or MRA should report it to the FDA by filing a MedWatch Adverse Event Report. Call 1-800-FDA-1088 or report via the FDA website at http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm
When filing a report online, remember that Gadolinium-Based Contrast Agents are considered prescription medications or drugs.
It is important that Adverse Event Reports related to Gadolinium-Based Contrast Agents are filed with the FDA or the full scope of Gadolinium-related health problems may never be brought to light.
Patients outside the U.S. report to their country’s equivalent governing agency.
Do you suffer from arthritis like Gena Norris? Learn real alternative treatment solutions to return to a pain-free life. The Arthritis Summit is playing online: Oct. 9-16, 2017. SIGN UP FOR FREE NOW.
This article is for informational purposes only and does not constitute medical advice. Read our full disclaimer here.
Recommended reading:
“This antibiotic will ruin you” – A Woman Had to Undergo 20 Surgeries to Repair Damage This Common Drug Caused. (FDA issued a warning too late…)
Do you suffer from arthritis like Gena Norris? Learn real alternative treatment solutions to return to a pain-free life. The Arthritis Summit is playing online: Oct. 9-16, 2017. SIGN UP FOR FREE NOW.
References
[1] Sherry, A. D., Caravan, P., & Lenkinski, R. E. (2009). Primer on gadolinium chemistry. Journal of Magnetic Resonance Imaging : JMRI, 30(6), 1240–8. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2853020&tool=pmcentrez&rendertype=abstract
[2] Gadolinium – Wikipedia, the free encyclopedia. (n.d.). Retrieved January 19, 2013, from http://en.wikipedia.org/wiki/Gadolinium
[3] MacDonald, N. S., & et al. (1951). The Skeletal Deposition of Yttrium. Journal of Biological Chemisgtr. Retrieved
from http://www.jbc.org/content/195/2/837.full.pdf
[4] Johannsson, O., Perrault, G., Savoie, L., & Tuchweber, B. (1968). Action of various metallic chlorides on calcaemia and phosphataemia. British Journal of Pharmacology and Chemotherapy, 33(1), 91–7. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1570281&tool=pmcentrez&rendertype=abstract
[5] Kindberg, G. M., Uran, S., Friisk, G., Martinsen, I., & Skotland, T. (2010). The fate of Gd and chelate following intravenous injection of gadodiamide in rats. European radiology, 20(7), 1636–43. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2882048/
[6] Gibby, W. A., Gibby, K. A., & Gibby, W. A. (2004). Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Investigative Radiology, 39(3), 138–42. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15076005
[7] White, G. W., Gibby, W. A., & Tweedle, M. F. (2006). Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investigative Radiology, 41(3), 272–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16481910
[8] Darrah, T. H., Prutsman-Pfeiffer, J. J., Poreda, R. J., Ellen Campbell, M., Hauschka, P. V, & Hannigan, R. E. (2009). Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics : integrated biometal science, 1(6), 479–88. Retrieved from http://pubs.rsc.org/en/content/articlehtml/2009/mt/b905145g
[9] HALEY, T. J., RAYMOND, K., KOMESU, N., & UPHAM, H. C. (1961). Toxicological and pharmacological effects of gadolinium and samarium chlorides. British Journal of Pharmacology and Chemotherapy, 17, 526–32. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1482085/?tool=pmcentrez&rendertype=abstract
[10] Spencer, A. J., Wilson, S. A., Batchelor, J., Reid, A., Pees, J., & Harpur, E. (1997). Gadolinium Chloride Toxicity in the Rat . Toxicologic Pathology , 25 (3 ), 245–255. doi:10.1177/019262339702500301 http://tpx.sagepub.com/content/25/3/245
[11] Rees, J., Spencer, A., Wilson, S., Reid, A., & Harpur, E. (1997). Time Course of Stomach Mineralization, Plasma, and Urinary Changes After a Single Intravenous Administration of Gadolinium(III) Chloride in the Male Rat . Toxicologic Pathology , 25 (6 ), 582–589. doi:10.1177/019262339702500607 http://tpx.sagepub.com/content/25/6/582.abstract
[12] Hirano, S., & Suzuki, K. T. (1996). Exposure, metabolism, and toxicity of rare earths and related compounds. Environmental health perspectives, 104 Suppl , 85–95. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1469566&tool=pmcentrez&rendertype=abstract
[13] Vassallo, D., & et al. (2011). Toxic effects of mercury, lead and gadolinium on vascular reactivity. Braz J Med Biol Res, 44, 939–946. Retrieved from http://www.scielo.br/pdf/bjmbr/v44n9/1088.pdf
[14] Husztik, E., Lázár, G., & Párducz, A. (1980). Electron microscopic study of Kupffer-cell phagocytosis blockade induced by gadolinium chloride. British Journal of Experimental Pathology, 61(6), 624–30. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2041618&tool=pmcentrez&rendertype=abstract
[15] Mizgerd, J., & et al. (1996). Gadolinium induces macrophage apoptosis. Journal of Leukocyte Biology, 59(February), 189–195. Retrieved from http://www.jleukbio.org/content/59/2/189.full.pdf
[16] Weinmann, H.-J., Brasch, R. C., Press, W.-R., & Wesbey, G. (1984). Characteristics of Gadolinium-DTPA Complex: A Potential NMR Contrast Agent. AJR. American ournal of roentgenology, March(142), 619–624. Retrieved from http://www.ajronline.org/content/142/3/619.full.pdf
[17] Brasch, R. C. (1985). Inherent contrast in magnetic resonance imaging and the potential for contrast enhancement. The 1984 L. Henry Garland lecture. The Western Journal of Medicine, 142(6), 847–53. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1306213&tool=pmcentrez&rendertype=abstract
[18] Mann, J. S. (1993). Stability of gadolinium complexes in vitro and in vivo. Journal of Computer Assisted Tomography, 17 Suppl 1, S19–23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8486827
[19] Rofsky, N. M., Sherry, A. D., & Lenkinski, R. E. (2008). Nephrogenic systemic fibrosis: a chemical perspective. Radiology, 247(3), 608–12. Retrieved from http://radiology.rsna.org/content/247/3/608.full
[20] Carr, D., & et al. (1984). Gadolinium-DTPA as a Contrast Agent in MRI: Initial Clinical Experience in 20 patients. AJR, August(143), 215–224. Retrieved from http://www.ajronline.org/content/143/2/215.full.pdf
[21] Cowper, S. E., Robin, H. S., Steinberg, S. M., Su, L. D., Gupta, S., & LeBoit, P. E. (2000). Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet, 356(9234), 1000–1. doi:10.1016/S0140-6736(00)02694-5 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11041404
[22] Grobner, T. (2006). Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrology, dialysis, transplantation : Official Publication of the European Dialysis and Transplant Association – European Renal Association, 21(4), 1104–8. Retrieved from http://ndt.oxfordjournals.org/
[23] Marckmann, P., Skov, L., Rossen, K., Dupont, A., Damholt, M. B., Heaf, J. G., & Thomsen, H. S. (2006). Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. Journal of the American Society of Nephrology : JASN, 17(9), 2359–62. doi:10.1681/ASN.2006060601 Retrieved from http://jasn.asnjournals.org/content/17/9/2359.full.pdf
[24] Abraham, J. L. (2011). Author Interview Jerrold L. Abraham, MD – Multiorgan Gadolinium Deposition and Fibrosis in a patient with Nephrogenic Systemic Fibrosis – an autopsy-based review. Hemodialysis.com. Retrieved August 15, 2012, from http://www.hemodialysis.com/author_interview_gd_deposition_in_pt_with_nsf.html
[25] Rocklage, S. M., Worah, D., & Kim, S.-H. (1991). Metal ion release from paramagnetic chelates: What is tolerable? Magnetic Resonance in Medicine, 22(2), 216–221. doi:10.1002/mrm.1910220211
[26] SUNY. (2012). Case 10 | Particle Analysis | SUNY Upstate Medical University. Retrieved September 30, 2012, from http://www.upstate.edu/pathenvi/studies/cases/case10.php
[27] Rofsky, N. M., Sherry, A. D., & Lenkinski, R. E. (2008). Nephrogenic systemic fibrosis: a chemical perspective. Radiology, 247(3), 608–12. Retrieved from http://radiology.rsna.org/content/247/3/608.full
[28] Sadowski, E. A., Bennett, L. K., Chan, M. R., Wentland, A. L., Garrett, A. L., Garrett, R. W., & Djamali, A. (2007). Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology, 243(1), 148–57. Retrieved from http://radiology.rsna.org/content/243/1/148.full
[29] Swaminathan, S., & Shah, S. V. (2007). New Insights into Nephrogenic Systemic Fibrosis. Journal of the American Society of Nephrology , 18 (10 ), 2636–2643. doi:10.1681/ASN.2007060645. Retrieved from http://jasn.asnjournals.org/content/18/10/2636.full.pdf+html
[30] Greenberg, S. A. (2010). Zinc transmetallation and gadolinium retention after MR imaging: case report. Radiology, 257(3), 670–3. Retrieved from http://radiology.rsna.org/content/257/3/670.full
[31] Cacheris, W. P., Quay, S. C., & Rocklage, S. M. (1990). The relationship between thermodynamics and the toxicity of gadolinium complexes. Magnetic Resonance Imaging, 8(4), 467–481. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2118207
[32] Salford, L. G., Nittby, H., & Persson, B. R. R. (2012). Effects of Electromagnetic Fields from Wireless Communication upon the Blood-Brain Barrier. BioInitiative Working Group (pp. 1–52, Section 10).Retrieved from http://www.bioinitiative.org/report/wp-content/uploads/pdfs/sec10_2012_Effects_Electromagnetic_Fields_Wireless_Communication.pdf
[33] Bayer HealthCare Pharmaceuticals. (2012). Magnevist Package Insert 2012.pdf. Retrieved October 14, 2012, from http://labeling.bayerhealthcare.com/html/products/pi/Magnevist_PI.pdf
[34] GE Healthcare. (2010). OMNISCAN Product Labeling. December2010. (020123s037lbl.pdf). Retrieved October 15, 2012, from http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020123s037lbl.pdf
[35] Mallinckrodt Inc. (2010). Optimark Product Labeling 2010. (020937s016lbl.pdf ). Retrieved October 15, 2012, from http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020937s016lbl.pdf
[36] Bracco Diagnostics Inc. (2010). ProHance Product Labeling 2010. (020131s024lbl.pdf ). Retrieved October 20, 2012, from http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020131s024lbl.pdf
[37] Bracco Diagnostics Inc. (2012). MultiHance Product Labeling 2012. 021357s011lbl.pdf. Retrieved October 11, 2012, from http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021357s011lbl.pdf
[38] DOTAREM-Product Information. (n.d.). Retrieved January 28, 2014, from https://gp2u.com.au/static/pdf/D/DOTAREM-PI.pdf
[39] Lantheus Medical Imaging. (2010). ABLAVAR Product Labeling 2010. (021711s003lbl.pdf). Retrieved October 15, 2012, from http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021711s003lbl.pdf
[40] Bayer HealthCare Pharmaceuticals. (2011). EOVIST Product Labeling 2011 (2011022090s005lbl.pdf). Retrieved October 15, 2012, from http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022090s005lbl.pdf
[41] Hesselink, J. R., Dunn, W. M., Healy, M. E., Hajek, P., Baker, L., & Brahme, F. J. (1986). Paramagnetic enhancement of cerebral lesions with gadolinium-DTPA. Acta radiologica. Supplementum, 369, 558–60. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2980556
[42] Martinez, R. (1995). Erdheim-Chester disease: MR of intraaxial and extraaxial brain stem lesions. American Journal of Neuroradiology , 16 (9 ), 1787–1790. Retrieved from http://www.ajnr.org/content/16/9/1787.full.pdf+html
[43] Tien, R. D., Brasch, R. C., Jackson, D. E., & Dillon, W. P. (1989). Cerebral Erdheim-Chester disease: persistent enhancement with Gd-DTPA on MR images. Radiology, 172(3), 791–2. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2772189
[44] Fulciniti, M., et al, & Dana Farber Cancer Institute Harvard Medical School. (2009). Gadolinium Containig Contrast Agent Promotes Multiple Myeloma Cell Growth: Implications for Clinical Use of MRI in Myeloma (poster presentation). Retrieved from http://myeloma.org/pdfs/ASH2009_Fulciniti_1809.pdf
[45] Xia, D., Davis, R. L., Crawford, J. A., & Abraham, J. L. (2010). Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiologica (Stockholm, Sweden : 1987), 51(10), 1126–36. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20868305
[46] Kanda, T., Ishii, K., Kawaguchi, H., Kitajima, K., & Takenaka, D. (2013). High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadolinium-based Contrast Material. Radiology, 131669. Retrieved from http://dx.doi.org/10.1148/radiol.13131669
[47] Cotton, F., Weiner, H. L., Jolesz, F. A., & Guttmann, C. R. G. (2003). MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology, 60(4), 640–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12601106
[48] Guttmann, C. R., Ahn, S. S., Hsu, L., Kikinis, R., & Jolesz, F. A. (1995). The evolution of multiple sclerosis lesions on serial MR. American Journal of Neuroradiology, 16(7), 1481–1491. Retrieved from http://www.ajnr.org/content/16/7/1481.full.pdf+html
[49] Sicotte, N. L. (2011). Neuroimaging in multiple sclerosis: neurotherapeutic implications. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics, 8(1), 54–62. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3075734/
[50] Simon, J. H. (1999). From enhancing lesions to brain atrophy in relapsing MS. Journal of neuroimmunology, 98(1), 7–15. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10426356
[51] Richert, N. D., Howard, T., Frank, J. A., Stone, R., Ostuni, J., Ohayon, J., Bash, C., et al. (2006). Relationship between inflammatory lesions and cerebral atrophy in multiple sclerosis. Neurology, 66(4), 551–6. Retrieved from http://www.neurology.org/content/66/4/551.abstract
[52] Bermel, R. A., & Bakshi, R. (2006). The measurement and clinical relevance of brain atrophy in mulitple sclerosis. Lancet Neurol, 5, 158–70. doi:10.1016/S1474-4422(06)70349-0 Retrieved from http://www.via.cornell.edu/ece578/project/2012/g6/Brain_atrophy.pdf
[53] Fulgenzi, A., & et al. (2012). A case of multiple sclerosis improvement following removal of heavy metal intoxication. Biometals. Retrieved from http://regenera.pt/documents/pages/A%20case%20of%20multiple%20sclerosis%20improvement%20following%20removal%20of%20heavy%20metal%20intoxication.pdf
[54] Tso, M. O. M., Shih, C.-Y., & McLean, I. W. (1975). Is There a Blood-Brain Barrier at the Optic Nerve Head? Archives of Ophthalmology, 93(9), 815–825. Retrieved from http://archopht.jamanetwork.com/article.aspx?articleid=631630
[55] Hofman, P., Hoyng, P., vanderWerf, F., Vrensen, G. F. J. M., & Schlingemann, R. O. (2001). Lack of Blood-Brain Barrier Properties in Microvessels of the Prelaminar Optic Nerve Head. Invest. Ophthalmol. Vis. Sci., 42(5), 895–901. Retrieved from http://www.iovs.org/cgi/content/abstract/42/5/895
[56] Maolood, N., & Meister, B. (2009). Protein components of the blood-brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. Journal of chemical neuroanatomy, 37(3), 182–95. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19146948
[57] Yokel, R. (2006). Blood-brain barrier flux of aluminum, manganese, iron and other metals suspected to contribute to metal-induced neurodegeneration. Journal of Alzheimer’s Disease, 10, 223–253. Retrieved from http://pharmacy.mc.uky.edu/faculty/files/jad.pdf
[58] Engelhardt, B., Wolburg-Buchholz, K., & Wolburg, H. (2001). Involvement of the choroid plexus in central nervous system inflammation. Microscopy research and technique, 52(1), 112–29. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11135454
[59] Starr, J. M., Wardlaw, J., Ferguson, K., MacLullich, A., Deary, I. J., & Marshall, I. (2003). Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. Journal of Neurology, Neurosurgery, and Psychiatry, 74(1), 70–6. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1738177&tool=pmcentrez&rendertype=abstract
[60] Kanamalla, U. S., & Boyko, O. B. (2002). Gadolinium Diffusion into Orbital Vitreous and Aqueous Humor, Perivascular Space, and Ventricles in Patients with Chronic Renal Disease . American Journal of Roentgenology , 179 (5 ), 1350–1352. Retrieved from http://www.ajronline.org/content/179/5/1350.short
[61] Boyko, O. B., Kanamalla, U. S., Memisoglu, E., Kochan, J., & Schulman, G. (2001). Flair Imaging of Gadolinium Diffusion into Brain CSF and Vitreous of the Globe in Patients with Renal Disease: Proton MRS and DWI Correlation. Retrieved from http://members.asnr.org/abstracts/2001/01-O-1007-ASNR.pdf
[62] Morris, J. M., & Miller, G. M. (2007). Increased signal in the subarachnoid space on fluid-attenuated inversion recovery imaging associated with the clearance dynamics of gadolinium chelate: a potential diagnostic pitfall. AJNR. American journal of neuroradiology, 28(10), 1964–7. Retrieved from http://www.ajnr.org/cgi/content/abstract/28/10/1964
[63] Mamourian, A. C., Jack Hoopes, P., & Lewis, L. D. (2000). Visualization of Intravenously Administered Contrast Material in the CSF on Fluid-AttenuatedInversion-Recovery MR Images: An In Vitro and Animal-Model Investigation . American Journal of Neuroradiology , 21 (1 ), 105–111. Retrieved from http://www.ajnr.org/content/21/1/105.abstract
[64] Kanamalla, U. S., Baker, K. B., & Boyko, O. B. (2001). Gadolinium Diffusion into Subdural Space . American Journal of Roentgenology , 176 (6 ), 1604–1605. Retrieved from http://www.ajronline.org/content/176/6/1604.short
[65] Guo, G., Wang, Q., Wang, J., Li, J., Ren, D., Zhen, L., Lin, H., et al. (2003). Effects of electromagnetic pulses on the blood-brain barrier of rats. Environmental Electromagnetics, 2003. CEEM 2003. Proceedings. Asia-Pacific Conference on 4-7 Nov. 2003. doi:10.1109/CEEM.2003.1282261. Retrieved from http://ieeexplore.ieee.org/xpl/login.jsp?reload=true&tp=&arnumber=1282261&url=http%253A
[66] Ding, G.-R., & et al. (2009). Effect of Electromagnetic Pulse Exposure on Brain Micro Vascular Permeability in Rats. Biomedical and environmental sciences BES, 22, 265–268. Retrieved from http://www.besjournal.com/Articles/Archive/2009/No3/201110/P020111014405434865643.pdf
[67] Ding, G.-R., Qiu, L.-B., Wang, X.-W., Li, K.-C., Zhou, Y.-C., Zhou, Y., Zhang, J., et al. (2010). EMP-induced alterations of tight junction protein expression and disruption of the blood-brain barrier. Toxicology letters, 196(3), 154–60. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20412840
[68] Zhou, J. X., Ding, G. R., Zhang, J., Zhou, Y. C., Zhang, Y. J., & Guo, G. Z. (2013). Detrimental effect of electromagnetic pulse exposure on permeability of in vitro blood-brain-barrier model. Biomedical and environmental sciences : BES, 26(2), 128–37. doi:10.3967/0895-3988.2013.02.007. Retrieved from http://www.besjournal.com/Articles/Archive/2013/No2/201301/t20130116_76358.html
[69] Salford, L. G., Nittby, H., Brun, A., Grafström, G., Malmgren, L., Sommarin, M., Eberhardt, J., et al. (2008). The Mammalian Brain in the Electromagnetic Fields Designed by Man with Special Reference to Blood-Brain Barrier Function, Neuronal Damage and Possible Physical Mechanisms. Progress of Theoretical Physics Supplement, 173(173), 283–309. Retrieved from http://ptps.oxfordjournals.org/content/173/283.full.pdf+html
[70] Nittby, H., Brun, A., Eberhardt, J., Malmgren, L., Persson, B. R. R., & Salford, L. G. (2009). Increased blood-brain barrier permeability in mammalian brain 7 days after exposure to the radiation from a GSM-900 mobile phone. Pathophysiology : the official journal of the International Society for Pathophysiology / ISP, 16(2-3), 103–12. Retrieved from http://www.pathophysiologyjournal.com/article/S0928-4680%252809%252900013-3/pdf
[71] Nittby, H., Grafström, G., Eberhardt, J. L., Malmgren, L., Brun, A., Persson, B. R. R., & Salford, L. G. (2008). Radiofrequency and extremely low-frequency electromagnetic field effects on the blood-brain barrier. Electromagnetic biology and medicine, 27(2), 103–26. doi:10.1080/15368370802061995. Retrieved from http://weepinitiative.org/LINKEDDOCS/health/nittby.PDF
[72] Naul, L. G., & Finkenstaedt, M. (1997). Extensive cerebrospinal fluid enhancement with gadopentetate dimeglumine in a primitive neuroectodermal tumor. American Journal of Neuroradiology, 18(9), 1709–1711. Retrieved from http://www.ajnr.org/content/18/9/1709.full.pdf+html
[73] Hamilton, B. E., & Nesbit, G. M. (2008). Delayed CSF enhancement in posterior reversible encephalopathy syndrome. AJNR. American journal of neuroradiology, 29(3), 456–7. Retrieved from http://www.ajnr.org/content/29/3/456.full
[74] Levy, L. M. (2007). Exceeding the limits of the normal blood-brain barrier: quo vadis gadolinium? AJNR. American journal of neuroradiology, 28(10), 1835–6. Retrieved from http://www.ajnr.org/content/28/10/1835.full
[75] Ray, D. E., & et al. (1996). Neurotoxic Effects of Gadopentetate Dimeglumine: Behavioral Disturbance and Morphology after Intracerebroventricular Injection in Rats. AJNR. American Journal of Neuroradiology, February(17), 365–373. Retrieved from http://www.ajnr.org/content/17/2/365.full.pdf
[76] Ray, D. E., Holton, J. L., Nolan, C. C., Cavanagh, J. B., & Harpur, E. S. (1998). Neurotoxic potential of gadodiamide after injection into the lateral cerebral ventricle of rats. . American Journal of Neuroradiology , 19 (8 ), 1455–1462. Retrieved from http://www.ajnr.org/content/19/8/1455.abstract
[77] Roman-Goldstein, S. M., Barnett, P. A., McCormick, C. I., Szumowski, J., Shannon, E. M., Ramsey, F. L., Mass, M., et al. (n.d.). Effects of Gd-DTPA after osmotic BBB disruption in a rodent model: toxicity and MR findings. Journal of computer assisted tomography, 18(5), 731–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8089321
[78] Hui, F., & Mullins, H. (2009). Persistence of Gadolinium Contrast Enhancement in CSF: A Possible Harbinger of Gadolinium Neurotoxicity? American Journal of Neuroradiology, January(30), E1. Retrieved from http://www.ajnr.org/content/30/1/e1.full.pdf
[79] Feng, X., Xia, Q., Yuan, L., Yang, X., & Wang, K. (2010). Impaired mitochondrial function and oxidative stress in rat cortical neurons: implications for gadolinium-induced neurotoxicity. Neurotoxicology, 31(4), 391–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20398695
[80] Xia, Q., Feng, X., Huang, H., Du, L., Yang, X., & Wang, K. (2011). Gadolinium-induced oxidative stress triggers endoplasmic reticulum stress in rat cortical neurons. Journal of neurochemistry, 117(1), 38–47. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21198628
[81] Nyman, U., Elmstahl, B., Leander, P., Nilsson, M., Golman, K., & Almen, T. (2002). Are Gadolinium-based Contrast Media Really Safer than Iodinated Media for Digital Subtraction Angiography in Patients with Azotemia? Radiology, 223(2), 311–318. Retrieved from http://radiology.rsna.org/content/223/2/311.full
[82] Heinrich, M. C., Kuhlmann, M. K., Kohlbacher, S., Scheer, M., Grgic, A., Heckmann, M. B., & Uder, M. (2007). Cytotoxicity of iodinated and gadolinium-based contrast agents in renal tubular cells at angiographic concentrations: in vitro study. Radiology, 242(2), 425–34. Retrieved from http://pubs.rsna.org/doi/full/10.1148/radiol.2422060245
[83] Akgun, H., Gonlusen, G., Cartwright Jr., J., Suki, W., & Truong, L. D. (2006). Are Gadolinium-Based Contrast Media Nephrotoxic? A Renal Biopsy Study. Arch Pathol Lab Med. Retrieved August 15, 2012, from http://www.archivesofpathology.org/doi/pdf/10.1043/1543-2165(2006)130%5B1354%3AAGCMNA%5D2.0.CO%3B2
[84] Thomsen, H. S., & et al. (2002). Gadolinium-containing Contrast Media for Radiographic Examinations: A Position Paper. European radiology, (12), 2600–2605. Retrieved from http://www.rbrs.org/dbfiles/journalarticle_0179.pdf
[85] Elmståhl, B., Nyman, U., Leander, P., Chai, C.-M., Golman, K., Björk, J., & Almén, T. (2006). Gadolinium contrast media are more nephrotoxic than iodine media. The importance of osmolality in direct renal artery injections. European Radiology, 16(12), 2712–20. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16896701
[86] Penfield, J. G., & Reilly, R. F. (2007). What nephrologists need to know about gadolinium. Nature Clinical Practice. Nephrology, 3(12), 654–68. Retrieved from http://dx.doi.org/10.1038/ncpneph0660
[87] Perazella, M. A. (2009). Current status of gadolinium toxicity in patients with kidney disease. Clinical journal of the American Society of Nephrology : CJASN, 4(2), 461–9. Retrieved from http://cjasn.asnjournals.org/content/4/2/461.long
[88] Sieber, M. A., Pietsch, H., Walter, J., Haider, W., Frenzel, T., & Weinmann, H.-J. (2008). A Preclinical Study to Investigate the Development of Nephrogenic Systemic Fibrosis: A Possible Role for Gadolinium-Based Contrast Media. Investigative Radiology, 43(1). Retrieved from http://journals.lww.com/investigativeradiology/Fulltext/2008/01000/A_Preclinical_Study_to_Investigate_the_Development.9.aspx
[89] Sieber, M. A., Lengsfeld, P., Frenzel, T., Golfier, S., Schmitt-Willich, H., Siegmund, F., Walter, J., et al. (2008). Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. European Radiology, 18(10), 2164–73. Retrieved from http://www.springerlink.com/content/1615m2x5700806k4/
[90] Pietsch, H., Pering, C., Lengsfeld, P., Walter, J., Steger-Hartmann, T., Golfier, S., Frenzel, T., et al. (2009). Evaluating the role of zinc in the occurrence of fibrosis of the skin: a preclinical study. Journal of magnetic resonance imaging : JMRI, 30(2), 374–83. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19629978
[91] Haylor, J., Dencausse, A., Vickers, M., Nutter, F., Jestin, G., Slater, D., Idee, J.-M., et al. (2010). Nephrogenic gadolinium biodistribution and skin cellularity following a single injection of Omniscan in the rat. Investigative Radiology, 45(9), 507–12. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20697223
[92] Haylor, J., Schroeder, J., Wagner, B., Nutter, F., Jestin, G., Idée, J.-M., & Morcos, S. (2012). Skin gadolinium following use of MR contrast agents in a rat model of nephrogenic systemic fibrosis. Radiology, 263(1), 107–16. Retrieved from http://pubs.rsna.org/doi/pdf/10.1148/radiol.12110881
[93] Gibby, W. A., Gibby, K. A., & Gibby, W. A. (2004). Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Investigative Radiology, 39(3), 138–42. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15076005
[94] Abraham, J. L., Thakral, C., Skov, L., Rossen, K., & Marckmann, P. (2008). Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. The British Journal of Dermatology, 158(2), 273–80. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18067485
[95] Christensen, K. N., Lee, C. U., Hanley, M. M., Leung, N., Moyer, T. P., & Pittelkow, M. R. (2011). Quantification of gadolinium in fresh skin and serum samples from patients with nephrogenic systemic fibrosis. Journal of the American Academy of Dermatology, 64(1), 91–6. doi:10.1016/j.jaad.2009.12.044
[96] Frenzel, T., Lengsfeld, P., Schirmer, H., Hütter, J., & Weinmann, H.-J. (2008). Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Investigative Radiology, 43(12), 817–28. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19002053
[97] Wermuth, P. J., Del Galdo, F., & Jiménez, S. A. (2009). Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis and Rheumatism, 60(5), 1508–18. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2737360&tool=pmcentrez&rendertype=abstract
[98] Varani, J., DaSilva, M., Warner, R. L., Deming, M. O., Barron, A. G., Johnson, K. J., & Swartz, R. D. (2009). Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Investigative radiology, 44(2), 74–81.
[99] Piera-Velazquez, S., Louneva, N., Fertala, J., Wermuth, P. J., Del Galdo, F., & Jimenez, S. A. (2010). Persistent activation of dermal fibroblasts from patients with gadolinium-associated nephrogenic systemic fibrosis. Annals of the Rheumatic Diseases, 69(11), 2017–23. Retrieved from http://ard.bmj.com/cgi/content/abstract/69/11/2017
[100] Del Galdo, F., Wermuth, P. J., Addya, S., Fortina, P., & Jimenez, S. A. (2010). NFκB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent Omniscan: possible role in the pathogenesis of nephrogenic systemic fibrosis. Annals of the Rheumatic Diseases, 69(11), 2024–33. Retrieved from http://ard.bmj.com/cgi/content/abstract/69/11/2024
[101] DaSilva, M., O’Brien Deming, M., Fligiel, S. E. G., Dame, M. K., Johnson, K. J., Swartz, R. D., & Varani, J. (2010). Responses of human skin in organ culture and human skin fibroblasts to a gadolinium-based MRI contrast agent: comparison of skin from patients with end-stage renal disease and skin from healthy subjects. Investigative Radiology, 45(11), 733–9. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3164303&tool=pmcentrez&rendertype=abstract
[102] Bleavins, K., Perone, P., Naik, M., Rehman, M., Aslam, M. N., Dame, M. K., Meshinchi, S., et al. (2012). Stimulation of fibroblast proliferation by insoluble gadolinium salts. Biological trace element research, 145(2), 257–67. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3273605&tool=pmcentrez&rendertype=abstract
[103] Bhagavathula, N., Dame, M. K., DaSilva, M., Jenkins, W., Aslam, M. N., Perone, P., & Varani, J. (2010). Fibroblast response to gadolinium: role for platelet-derived growth factor receptor. Investigative Radiology, 45(12), 769–77. Retrieved from http://europepmc.org/articles/PMC3164279;jsessionid=i8I095NYY5uIueGBg1pw.22
[104] Edward, M., Quinn, J. A., Burden, A. D., Newton, B. B., & Jardine, A. G. (2010). Effect of different classes of gadolinium-based contrast agents on control and nephrogenic systemic fibrosis-derived fibroblast proliferation. Radiology, 256(3), 735–43. Retrieved from http://radiology.rsna.org/content/256/3/735.full
[105] Tweedle, M. F., Wedeking, P., & Kumar, K. (1995). Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Investigative Radiology, 30(6), 372–80. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7490190
[106] Aime, S., & Caravan, P. (2009). Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. Journal of Magnetic Resonance Imaging, 30(6), 1259–1267. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2822463&tool=pmcentrez&rendertype=abstract
[107] Wollanka, H., Weidenmaier, W., & Giersig, C. (2009). NSF after Gadovist exposure: a case report and hypothesis of NSF development. Nephrology Dialysis Transplantation , 24 (12 ), 3882–3884. Retrieved from http://ndt.oxfordjournals.org/content/24/12/3882.full.pdf+html
[108] Elmholdt, T. R., Jorgensen, B., Ramsing, M., Pedersen, M., & Olesen, A. B. (2010). Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus, 3(3), 285–287. Retrieved from http://ckj.oxfordjournals.org/content/3/3/285.full
[109] Sanyal, S., Marckmann, P., Scherer, S., & Abraham, J. (2011). Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis – an autopsy-based review. Nephrology Dialysis Transplant, (0), 1–11. Retrieved from http://ndt.oxfordjournals.org/content/early/2011/03/25/ndt.gfr085.full.pdf
[110] Ting, W. W., Stone, M. S., Madison, K. C., & Kurtz, K. (2003). Nephrogenic fibrosing dermopathy with systemic involvement. Archives of Dermatology, 139(7), 903–6. Retrieved from http://archderm.jamanetwork.com/article.aspx?articleid=479394
[111] Aggarwal, A., Froehlich, A. A., Essah, P., Brinster, N., High, W. A., & Downs, R. W. (2011). Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. Journal of Medical Case Reports, 5, 566. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3253728&tool=pmcentrez&rendertype=abstract
[112] Gibson, S., Farver, C., & Prayson, R. (2006). Multiorgan Involvement in Nephrogenic Fibrosing Dermopathy An Autopsy Case and Review of the Literature. Gibson SE, Farver CF, Prayson RA. Arch Pathol Lab Med. 2006;130:209–212. Arch Pathol Lab Med, (130), 209–212. Retrieved from http://www.archivesofpathology.org/doi/pdf/10.1043/1543-2165%282006%29130%5B209%3AMIINFD%5D2.0.CO%3B2
[113] Daram, S. R., Cortese, C. M., & Bastani, B. (2005). Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. American Journal of Kidney Diseases : the official journal of the National Kidney Foundation, 46(4), 754–9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16183432
[114] Krous, H. F., Breisch, E., Chadwick, A. E., Pinckney, L., Malicki, D. M., & Benador, N. (2007). Nephrogenic systemic fibrosis with multiorgan involvement in a teenage male after lymphoma, Ewing’s sarcoma, end-stage renal disease, and hemodialysis. Pediatric and Developmental Pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society, 10(5), 395–402. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17929984
[115] Kucher, C., Steere, J., Elenitsas, R., Siegel, D. L., & Xu, X. (2006). Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. Journal of the American Academy of Dermatology, 54(2 Suppl), S31–4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16427988
[116] Swaminathan, S., High, W. A., Ranville, J., Horn, T. D., Hiatt, K., Thomas, M., Brown, H. H., et al. (2008). Cardiac and vascular metal deposition with high mortality in nephrogenic systemic fibrosis. Kidney International, 73(12), 1413–8. Retrieved from http://dx.doi.org/10.1038/ki.2008.76
[117] Koreishi, A., & et al. (2009). Nephrogenic Systemic Fibrosis A Pathologic Study of Autopsy Cases. Arch Pathol Lab Med, 133(December), 1943–1948. Retrieved from http://www.archivesofpathology.org/doi/pdf/10.1043/1543-2165-133.12.1943
[118] Saenz, A., Mandal, R., Kradin, R., & Hedley-Whyte, E. T. (2006). Nephrogenic fibrosing dermopathy with involvement of the dura mater. Virchows Archiv : an international journal of pathology, 449(3), 389–91. Retrieved from http://link.springer.com/article/10.1007%2Fs00428-006-0246-x
[119] Perez-Rodriguez, J., Lai, S., Ehst, B. D., Fine, D. M., & Bluemke, D. A. (2009). Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment–report of 33 cases. Radiology, 250(2), 371–7. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2663356&tool=pmcentrez&rendertype=abstract
[120] Barker-Griffith, A., Goldberg, J., & Abraham, J. L. (2011). Ocular pathologic features and gadolinium deposition in nephrogenic systemic fibrosis. Archives of Ophthalmology, 129(5), 661–3. Retrieved from http://archopht.jamanetwork.com/article.aspx?articleid=427337
[121] Jornet, A. R. et al. (2009). Revista Nefrologia – Gadolinium-induced systemic fibrosis in severe renal failure. Revista Nefrologia. Retrieved September 28, 2012, from http://www.revistanefrologia.com/modules.php?name=articulos&d_op=&idarticulo=129&idlangart=EN&preproduccion=
Find more info at https://gadoliniumtoxicity.com/